Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.49 n.1 San José Mar. 2001
J. P. Lewis 1; S. L. Stofella 2 and S. R. Feldman 1
Recibido 19-II-2000. Corregido 3-VII-2000. Aceptado 31-VII-2000
Abstract
Monk's tonsure-like gaps develop inside gramineans and other plants. The tonsures of Spartina argentinensis originate as a result of tussock development and disturbance. As the tonsure develops the ring of tillers around it breakes down and new tussocks develop from the fragments, regenerating the grassland matrix vegetatively. The microenvironment inside the tonsure is different from the surroundings and microhabitat-specific taxa grow there.
Key words: Chaco, gaps, grasses, grasslands, matrix regeneration, pampa, disturbance, regeneration niche, savannas, Spartina argentinensis, tussocks
In a previous paper (Lewis et al. 1990, b) we reported the presence of monk's tonsure-like gaps in tussocks of Spartina argentinensis Parodi, 1919, and Elionurus muticus (Spreng.) Kuntze, 1898, in the Submeridional Lowlands of the Austral Chaco region. We have also seen this type of structure in other grasses such as Paspalum quadrifarium Lamarck, 1791, Stipa brachychaeta Godron, 1853, Spartina densiflora Brongniart in Du Perry, 1829 and Leptochloa chloridiformis (Hack.) Parodi, 1918, in the Chaco and Pampa regions.
Similar structures have been observed earlier by Watt (1947, 1955) in Festuca ovina L. and non grasses such as Calluna vulgaris Salisb. in England and Ruiz-Leal (1959), quoted more than 30 species, mostly grasses, with this type of gaps in the Andes. Muhlenbergia torreyi Kunth (Hitchcock) ex Bush vernaculars name, "ring muhly", refers to the existance of gaps. However, monk's tonsure like gaps have been almost overlooked in scientific literature (Strickland 1983).
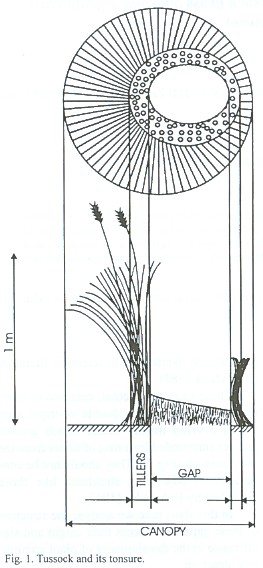
These gaps are circular, centered or more often excentric; single, double or triple gaps which develop mainly inside bunch grasses and are surrounded by a ring of tillers from the same tussock (Fig. 1). They should not be confused with other ring structures like those described by Rohmeder (1947).
In this short note we analyse the structure of these gaps and discuss their origin and significance in the development of plant community structure.
S. argentinensis grasslands and savannas are very widespread in the Submeridional Lowlands of the Chaco and other depressed areas of the chaco-pampean plain, and have already been described at length (Lewis et al. 1990 a). We visited several stands of the community surveying tussocks with this type of gap. We measured and made detailed diagrams of several tussocks, dug them up to analyze morphology and collected soil samples inside the gap and in the inter-tussock space. Plants were seeded in pots and growth and development of the tussocks was recorded.
Sizes and relative position of the gap are variable, but in all cases the soil inside the tussock is 10 to 20 cm higher than outside. The gap soil is more friable and deeper than the inter-tussock soil and organic matter, charcoal and ashes accumulate in the tonsure. Carbon (3.8-5.1 % inside the gap; 1.9-3.2 % outside) and organic matter (6.6-8.8 % inside the gap; 3.3-5.4 % outside) are more concentrated in the tonsures and pH (5.6-6.8 inside; 7.2-7.7 outside) is slightly more acid inside the gaps. Phosphate and calcium concentration are higher while sodium is slightly lower inside the gap.
The tussocks are formed by radial units that we name buttresses. Each is a short rhizome that grows centrifugally and when the center dies buttresses become independent of each other. On the outside, rhizomes sometimes produce two buds that generate buttresses of 2nd, 3rd or higher order that eventually may become independent. When some or all the buttresses from one side die the tussock becomes semilunar and the remaining buttresses grow away from the tussock's focus. Eventually a bud may grow regenerating the tussock's round shape. Few buttresses or tillers (sometimes only one) are needed to regenerate a whole tussock, but in these cases young tussocks will be oval-shaped. When a tussock originates from a seed, the core dies early and buttresses become independent of each other.
Several hypotheses can be advanced on the origin of this type of gap (Ruiz-Leal 1959, Strickland 1983), but none of them explain all the cases. Perhaps a different hypothesis will be needed for each species or locality. Nevertheless it seems that the gaps are formed as a consequence of development or as a result of some sort of disturbance like fire or overgrazing. In our case in these gaps become apparent in tussocks larger than a certain size and charcoal and ashes accumulate in them; also Camponotus punctulatus (Mayr.) ant-hills, which are common in the region (Pire et al. 1991), are sometimes built in the center of tussocks. Therefore we can assume that these disturbances may contribute to the creation of some of these gaps. However, in seed-grown plants, the core dies at a very early stage, so this fact suggests that the tussock development is the primary cause for monks tonsure like gap formation.
If in the field these gaps are not apparent at an early stage it is because they are filled with dead culms and leaves that cover the gap. Once a fire burns all these debris out, the tussock resprouts and the monks tonsure becomes apparent.
The importance of canopy gaps in the maintenance of species richness of forests and grasslands has already been recognized (Miles 1972, Fenner 1978, Denslow 1980, Gross 1980, Picket 1980, 1982, Whitmore 1982). Light could be as much as 60 times more intense in a monk's tonsure like gap at noon on summer sunny days than under a close dense canopy of S. argentinensis (pers. obs.). Soil characteristics are completely different inside the gaps, therefore they are specialized microsites and can be the regeneration niche (Grubb 1977) or a safe site (Harper 1977) for germination of certain species. In the Submeridional Lowlands, Desmanthus chacoensis Burck, 1952, is very frequent inside the tonsures, but very rare elsewhere, while other species like Aster squamatus (Spreng.) Hieronymus, 1901, rather frequent in Spartina grasslands, never appear inside the gaps (pers. obs.).
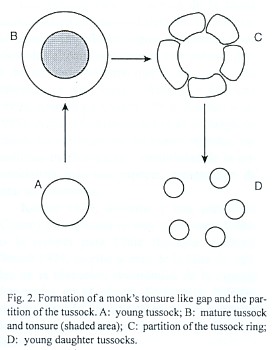
When a tussock grows the tonsure becomes larger and eventually the ring of tillers is broken down in several parts (Fig. 2). At least some of the ring fragments generate new tussocks. Therefore the tonsures development is a major contribution to the vegetative regeneration of the community matrix. The dynamics of tonsure-like gap formation in the Chaco region and elswhere deserves further study.
AcknowledgmentsIn a previous paper (Lewis et al. 1990, b) we reported the presence of monk's tonsure-like gaps in tussocks of Spartina argentinensis Parodi, 1919, and Elionurus muticus (Spreng.) Kuntze, 1898, in the Submeridional Lowlands of the Austral Chaco region. We have also seen this type of structure in other grasses such as Paspalum quadrifarium Lamarck, 1791, Stipa brachychaeta Godron, 1853, Spartina densiflora Brongniart in Du Perry, 1829 and Leptochloa chloridiformis (Hack.) Parodi, 1918, in the Chaco and Pampa regions.
Similar structures have been observed earlier by Watt (1947, 1955) in Festuca ovina L. and non grasses such as Calluna vulgaris Salisb. in England and Ruiz-Leal (1959), quoted more than 30 species, mostly grasses, with this type of gaps in the Andes. Muhlenbergia torreyi Kunth (Hitchcock) ex Bush vernaculars name, "ring muhly", refers to the existance of gaps. However, monk's tonsure like gaps have been almost overlooked in scientific literature (Strickland 1983).
These gaps are circular, centered or more often excentric; single, double or triple gaps which develop mainly inside bunch grasses and are surrounded by a ring of tillers from the same tussock (Fig. 1). They should not be confused with other ring structures like those described by Rohmeder (1947).
In this short note we analyse the structure of these gaps and discuss their origin and significance in the development of plant community structure.
S. argentinensis grasslands and savannas are very widespread in the Submeridional Lowlands of the Chaco and other depressed areas of the chaco-pampean plain, and have already been described at length (Lewis et al. 1990 a). We visited several stands of the community surveying tussocks with this type of gap. We measured and made detailed diagrams of several tussocks, dug them up to analyze morphology and collected soil samples inside the gap and in the inter-tussock space. Plants were seeded in pots and growth and development of the tussocks was recorded.
Sizes and relative position of the gap are variable, but in all cases the soil inside the tussock is 10 to 20 cm higher than outside. The gap soil is more friable and deeper than the inter-tussock soil and organic matter, charcoal and ashes accumulate in the tonsure. Carbon (3.8-5.1 % inside the gap; 1.9-3.2 % outside) and organic matter (6.6-8.8 % inside the gap; 3.3-5.4 % outside) are more concentrated in the tonsures and pH (5.6-6.8 inside; 7.2-7.7 outside) is slightly more acid inside the gaps. Phosphate and calcium concentration are higher while sodium is slightly lower inside the gap.
The tussocks are formed by radial units that we name buttresses. Each is a short rhizome that grows centrifugally and when the center dies buttresses become independent of each other. On the outside, rhizomes sometimes produce two buds that generate buttresses of 2nd, 3rd or higher order that eventually may become independent. When some or all the buttresses from one side die the tussock becomes semilunar and the remaining buttresses grow away from the tussock's focus. Eventually a bud may grow regenerating the tussock's round shape. Few buttresses or tillers (sometimes only one) are needed to regenerate a whole tussock, but in these cases young tussocks will be oval-shaped. When a tussock originates from a seed, the core dies early and buttresses become independent of each other.
Several hypotheses can be advanced on the origin of this type of gap (Ruiz-Leal 1959, Strickland 1983), but none of them explain all the cases. Perhaps a different hypothesis will be needed for each species or locality. Nevertheless it seems that the gaps are formed as a consequence of development or as a result of some sort of disturbance like fire or overgrazing. In our case in these gaps become apparent in tussocks larger than a certain size and charcoal and ashes accumulate in them; also Camponotus punctulatus (Mayr.) ant-hills, which are common in the region (Pire et al. 1991), are sometimes built in the center of tussocks. Therefore we can assume that these disturbances may contribute to the creation of some of these gaps. However, in seed-grown plants, the core dies at a very early stage, so this fact suggests that the tussock development is the primary cause for monks tonsure like gap formation.
If in the field these gaps are not apparent at an early stage it is because they are filled with dead culms and leaves that cover the gap. Once a fire burns all these debris out, the tussock resprouts and the monks tonsure becomes apparent.
The importance of canopy gaps in the maintenance of species richness of forests and grasslands has already been recognized (Miles 1972, Fenner 1978, Denslow 1980, Gross 1980, Picket 1980, 1982, Whitmore 1982). Light could be as much as 60 times more intense in a monk's tonsure like gap at noon on summer sunny days than under a close dense canopy of S. argentinensis (pers. obs.). Soil characteristics are completely different inside the gaps, therefore they are specialized microsites and can be the regeneration niche (Grubb 1977) or a safe site (Harper 1977) for germination of certain species. In the Submeridional Lowlands, Desmanthus chacoensis Burck, 1952, is very frequent inside the tonsures, but very rare elsewhere, while other species like Aster squamatus (Spreng.) Hieronymus, 1901, rather frequent in Spartina grasslands, never appear inside the gaps (pers. obs.).
When a tussock grows the tonsure becomes larger and eventually the ring of tillers is broken down in several parts (Fig. 2). At least some of the ring fragments generate new tussocks. Therefore the tonsures development is a major contribution to the vegetative regeneration of the community matrix. The dynamics of tonsure-like gap formation in the Chaco region and elswhere deserves further study.
Acknowledgments
We thank Cecilia Vieto for her help with the figures.
Resumen
Los claros tipo tonsura de monje se desarrollan tanto en el interior de matas de gramíneas, como de especies no pertenecientes a dicha familia. Describimos las matas de Spartina argentinensis y sus tonsuras que surgen por el propio desarrollo de la mata y disturbios. A medida que la tonsura se desarrolla, el anillo de culmos que la rodea se rompe y nuevas matas se desarrollan a partir de los fragmentos, regenerando vegetativamente la matriz del pastizal. Los microambientes dentro y fuera de la tonsura son distintos, al igual que las especies que se establecen en ellos.
Referencias
Denslow J.S. 1980. Gap partitioning among tropical rainforest trees. Biotropica 12 (Suppl.): 47-55. [ Links ]
Fenner M. 1978. A comparison of the abilities of colonizers and close-turf species to establish from seeds in artificial swards. J. Ecol. 66: 953-963. [ Links ]
Gross K. L. 1980. Colonization by Verbascum thapsus (Mullein) of an old field in Michigan: Experiments on th effect of vegetation. J. Ecol 68: 919-927. [ Links ]
Grubb P. J. 1977. The maintenance of species-richness in plant communities: the importance of the regeneration niche. Bio. Rev 52: 107-145. [ Links ]
Harper J. L. 1977. Population Biology of Plants. Academic, New York. [ Links ]
Lewis J. P.; E. F. Pire; D. E. Prado; S. L. Stofella; E. A. Franceschi & N. J. Carnevale. 1990 a.Plant communities and phytogeographical position of a large depression in the Great Chaco, Argentina. Vegetatio 86: 25-38.
Lewis J. P.; S.L. Stofella; D. E. Prado; E. F. Pire; E. A. Franceschi & N. J. Carnevale. 1990 b. Dynamics and development of floristic richness in the vegetation of a large depressed area of the Great Chaco. Flora 184: 63-77.
Miles J. 1972. Experimental establishment of seedlings on southern English heath. J. Ecol. 60: 225-234. [ Links ]
Pickett S. T. A. 1980. Non-equilibrium coexistence of plants. Bull. Torrey Bot. Club 107: 238-248. [ Links ]
Pickett S. T. A. 1982. Population patterns through twenty years old field succession. Vegetatio 49: 49-59. [ Links ]
Pire E. F.; P.S. Torres; O. D. Romagnoli & J. P. Lewis. 1991. The significance of ant-hills in depressed areas of the Great Chaco. Rev. Biol. Trop. 39: 71-76. [ Links ]
Rohmeder G. 1947. Investigación fitoestadística en un erial patagónico. An. Soc. Arg. Est. Geog. GAEA 8: 73-80. [ Links ]
Ruiz-Leal A. 1959. El desarrollo de estructuras subcirculares en algunas plantas. Rev. Agron. NO. Arg. 3: 83-138.
Strickland R. 1983. Hollow crowns: Overgrazing, undergrazing, or old age? Rangelands 5: 13-14. [ Links ]
Watt A. S. 1947. Pattern and process in the plant community. J. Ecol. 35: 1-22. [ Links ]
Watt A. S. 1955. Bracken versus heather, a study in plant sociology. J. Ecol. 43: 490-506. [ Links ]
Whitmore T. C. 1982. On pattern and process in forests. In E. I. Newman (ed.) The plant community as a working mechanism.. Blackwell, Oxford. pp 45-59.
1 Facultad de Ciencias Agrarias, Universidad Nacional de Rosario, CC 14, 2123 Zavalla, fax 54 -341–4970199, sfeldman@sede.unr.edu.ar;
2 Centro de Ecofisiología Vegetal, Buenos Aires, Argentina.














