Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.49 n.1 San José Mar. 2001
Recibido 21-XI-1999. Corregido 27-VII-2000. Aceptado 31-VII-2000.
Abstract
One of the objectives of the Sierra de Manantlán Biosphere Reserve (Jalisco, México) is the conservation in situ of the teosinte Zea diploperennis Iltis, Doebley, Guzman & Pazzi. Zea diploperennis is perennial, shade intolerant and its 1-3 m shoots are architecturally similar to maize. Clonal growth is of the phalanx type. Genets are iteroparous (modules semelparous). The demography of seven module and genet populations was studied in seven sites representing three stages of old-field succession. Seven permanent one-meter-square plots were randomly established in each site. All genets initially present and those that became established during our study were mapped and labeled according to year of establishment The magnitude of demographic fluctuations was greater in module populations. Genet population dynamics followed a seasonal rhythm with a maximum population size obtained at the onset of the rainy season. A relation was documented between percent annual mortality of a cohort and its age: the younger the cohort, the greater the mortality. This was a statistically significant relationship, Y=[ sin(-0.288x + 1.657)]2 (r = 0.92, p < 0.01), where is proportion annual mortality of genets and is the age of the cohort. The maximum rates of genet mortality occurred during the rainy season when population densities were greatest. As a consequence, we postulate that competition occurs principally during the rainy season. Linear relationships were observed between rate of population increase of genets versus old-field successional stage and soil type. Those areas with poor soil (Ultisol), degraded soil or soils with similar physical characteristics could be rehabilitated by introducing Z. diploperennis. Such rehabilitation would achieve two distinct objectives, promote propagation of this rare endemic and reclaim areas that are susceptible to erosion and further degradation.
Key words: Initial seedling recruitment, Manantlán, México, population structure, rehabilitation, teosinte, Zea diploperennis.
Study of an organisms basic ecology is the first step in conservation planning and decision-making. Such research provides a basis for understanding and documenting critical stages of a species life history. Protecting and maintaining rare and threatened species in their natural habitats is one of the goals of conservation biology (Lesica 1992) and of the Biosphere Reserves in the Man and the Biosphere – United Nations Educational, Scientific and Cultural Organization program (MAB-UNESCO) (Halffter 1988). Species rarity may be caused by evolutionary and historical events that resulted in the present-day distributions, or by existing ecological and genetic factors (Watson et al. 1994). Investigating the autoecology and natural history of rare species is one area in which ecologists can apply their experience to current problems in conservation biology or recovery plans (Harvey 1985, Brussard 1991, Boyce 1992, Huenneke 1995, Tear et al. 1995). However, to be successful, this outlook must be applied in long-term conservation programs. Demographic monitoring of rare plant species is an essential component of an effective species management program (Gilpin & Soulé 1986, Owen & Rosentreter 1992, Olmsted & Alvarez-Buylla 1995) in which active manipulations might be part of such a program (Boyce 1992).
One of the objectives of the Sierra de Manantlán Biosphere Reserve is the conservation in situ of the teosinte Zea diploperennis (Jardel 1992), a rare endemic species narrowly restricted in geographic range to a few hundred hectares (Rabinowitz et al. 1986). In spite of the fact that teosintes (wild Zea L. Gramineae) are a potential source of economically valuable germoplasm for maizes improvement (Iltis et al. 1979, Hoyt 1988, Sánchez-Velásquez 1991, Wilkes 1994), their basic population ecology has not been studied under local natural conditions. Zea diploperennis is a tropical endemic species occurring naturally in patches on approximately 360 hectares in the Sierra de Manantlán Biosphere Reserve in Jalisco, México (Benz et al. 1990). Growth and development appear to be favored by traditional forms of slash and burn agriculture (Sánchez-Velásquez et al. 1991). Zea diploperennis is a perennial, shade intolerant, rhizomatous grass whose one to three meter shoots are architecturally similar to maize. Clonal growth is of the phalanx type (Hutchings & Bradbury 1986) where modules (epigeal shoots arising from underground hypogeal rhizome) do not separate themselves from the parent shoot. Genets are iteroparous (but their modules are semelparous), reach sexual maturity and are capable of developing more than one module in the first two years (Sánchez-Velásquez & Lorente-Adame 1993, Lorente-Adame & Sánchez-Velásquez 1996).
This paper reports the descriptive phase of a long-term study whose ultimate goal is to implement a management program to conserve Z. diploperennis in situ. We describe the structure and population flux of genets and modules of seven populations of Z. diploperennis. We also evaluated the relationship between various environmental factors (altitude, exposure, slope, and soil type) and the stage of old field succession, and demographic attributes (rate of increase of genets and modules and genets arrival). In addition, we explored the relation between age and rate of mortality genet.
Materials and methods
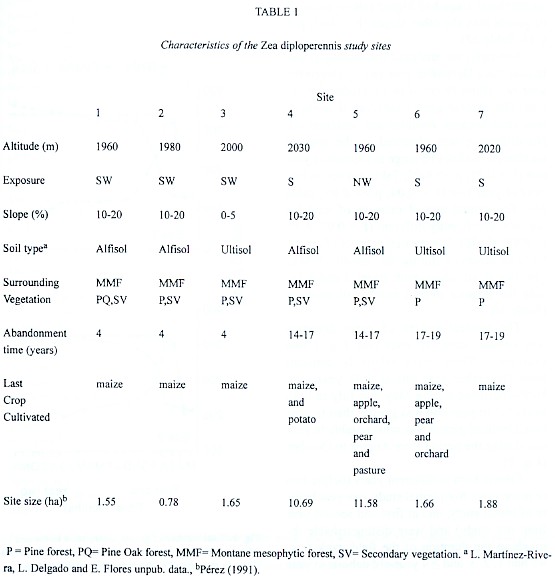
The seven study sites are located in the Las Joyas Scientific Station in the Sierra de Manantlán Biosphere Reserve in Jalisco, México (19°36"N, 104°16'W). Grazing and forest fires have been excluded since 1988 from all sites. All sites are old abandoned maize fields; these are: Coamil 1 (Site 1), Coamil 2 (Site 2), Playas (Site 3), Huiscorol (Site 4), Zarzamoro (Site 5), Asoleaderos (Site 6) and Asoleaderos del Tlacuache (Site 7), which represent three different time intervals since abandonment, i.e. different successional stages (Table 1). The characteristics of each site and its history are listed in Table 1.
Seven permanent one-meter square plots were randomly established in each of the seven abandoned fields. All genets present initially and those that became established subsequently were mapped and labeled according to the year of establishment (cf. Sarukhán & Harper 1973). The number of modules per genet was recorded monthly from May to December during year one and year two (except for June and September). In May-year one we could differentiate the genets which were born in one past year because they had one, or rarely two, small modules and one or no inflorescence. Cohorts born during our study were could identified easily: in the first-year, the first leaves to emerge have emarginate apices and in following years have an acuminate apex. Births and deaths were recorded according to age and comparisons of the 1, 2, 3 and > 4 year-old cohorts were performed. The number of genets established by themselves, the total number of genets and modules (initial and final), and rate of increase of genets and modules, were compared according to successional stage and environmental factors (altitude, exposure, slope and soil type) using procedure two factors general linear model (GLM) with values previously transformed to ranks (except rate of increase of modules and genets) and a posteriori, multiple comparisons using Tukey´s test from Statistic Analysis Systems (SAS) version 6.03 (Seaman et al. 1994). In this case, performing analysis of variance (ANOVA) with values transformed to ranks is more conservative than the corresponding ANOVA without transformation and is frequently more accurate than the non parametric Kruskal-Wallis test (SAS 1988). In addition, we used a general linear model procedure to obtain the relationship between proportion annual mortality of genets (these values were previously transformed to their square root and then arcsine were calculated) and age cohorts.
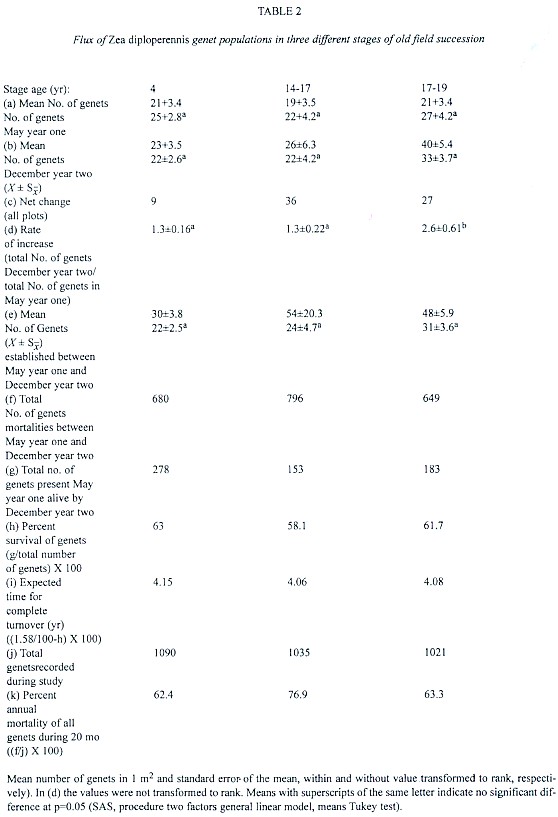
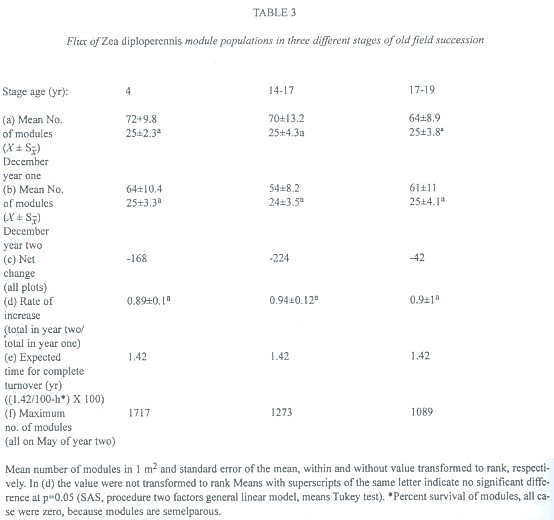
Population flux of genets was analyzed during two reproductive cycles from May (year one) to December (year two). The initial number of genets (May year one) did not differ significantly among successional stages (F = 0.31, p >> 0.05), nor was there a significant difference among the final number of genets (December year two; F = 3.2, p > 0.05; Table 2). Furthermore, the difference in the recruitment between them was not significant during May year one and December year two (F = 2.13, p > 0.05; Table 2e). However, the oldest successional stage had higher rate of increase of genets than the other stages (F = 4.65, p < 0.05; Table 2d).
Similarly, we analyzed the module populations from December year one to December year two (final phase of two reproductive cycles).The percent age of survival of modules was zero because modules are semelparous. No difference was observed in the average number of modules by stage in December year one (F = 0.01, p >> 0.05; Table 3a) nor in December year two (F = 0.04, p >> 0.05; Table 3b). The annual rate of increase of modules was not significantly different (F = 0.07, p >> 0.05)(Table 3d). The greatest number of modules was observed during the month of May year two in all stages, with stages 1 and 2 having the greatest overall number of modules (Table 3f).
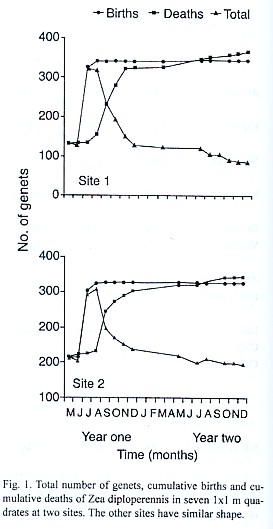
The general pattern of genet densities was similar across all sites (see Fig. 1 two examples). These were relatively constant from December year one to July year two. Births occurred during May and July in both years but in year one was greater than in year two. Death of genets was most notable in year one during the period from August to October (Fig. 1).
Genets born in different years (before two or more years from initial study, one year before of initial study, and at first and second year from our study) and were distinguishable throughout the duration of the field work (i.e., we had 1, 2, 3, and > 4 year-old cohorts in year two final). The highest genet mortality rates occurred from August to October and were greatest for cohorts were born in year one (Fig. 2a). When young genets initiated growth, more than 50 percent appeared chlorotic and died by damping-off. The remainder disappeared from areas excavated by gophers and due to unknown causes. Genets born before two or more years from initial study showed a greatest mortality from July to October in both years (Fig. 2a). Genets mortality appeared to be relatively more stable for all cohorts as time passed.
Patterns of module population fluctuations were similar for cohorts of the same age across sites. The number of modules from genets were born in year one was similar to genet survival curves of the same age probably because genets generally begin with a single module. The greatest number of modules was recorded during the months of July from year one and May from year two, and slowly decreased from August to November. The cohort born in year two contributed with a relatively lower number of genets and modules than the previous cohorts (Fig. 2b).
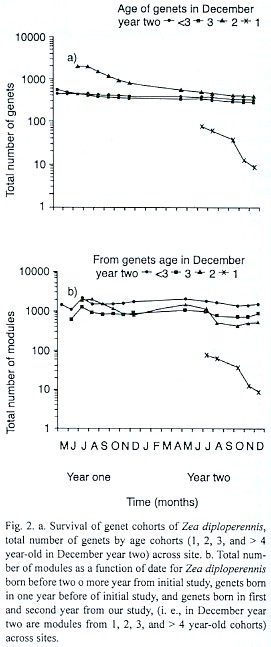
Genets born in year one and before were most abundant in sites one, two, three, four, five six and seven (Table 4). The most abundant cohorts at site three were genets of two and three year old. Recruitment during year two was zero at sites two, four and five, with the other sites showing very low recruitment. Proportion mortality (from May year one through December year two) among age cohorts was significantly different (F = 123.4, p << 0.01). The relationship between proportion of genets mortality and age was estimated as: Y= sin(-0,288X + 1.657)2 , where is the proportion of mortality and is the age of the cohort, was significant (r = 0.92, p<< 0.01). Soil type explained significant differences in genets increase rate (Ultisol > Alfisol, 2.31 + 0.4 and 1.2 + 0.1; genets in December year two/genets in May year one respectively; F=8.64, p< 0.01).
Discussion
Only two partial associations were observed, between rate of population increase of genets versus type of soil and stage old field succession (Table 2), the genets increase rate was higher in the oldest stage and Ultisols than in younger stages and Alfisols. Ultisols have low fertility and low base content (Boul et al. 1993). Our results suggest that Z. diploperennis has greater adaptive success in Ultisols than in Alfisols, hence the higher genets increase rate in Ultisols.
In all stages of abandonment genets increase rate was greater than one. These rates resulted in high recruitment in year one. Nevertheless, when we estimated genets increase rate from December year one to December year two, the values were lower than one (see Tables two and four). Our results suggest that it is important to monitor genet flux for a greater length of time because there was high variation in density during both years. Thus appropriate models of population dynamics must be based on numerous years of monitoring in order to adequately conduct projection and population viability analysis.
No trends between the successional stage or environmental factors (altitude, exposure, slope and soil type) versus module population parameters were observed (Table 3). Our results differ from Benz et al. (1990) who worked with the same species yet demonstrated a significant positive relationship between number of modules and age stages. Perhaps their trend is a result of having included three sites in each of two localities differing in management history: three sites in San Miguel with very young stages and three others with old stages in Las Joyas.
In our study, the net change of modules was negative for all stages (Table 3c). The oldest stage had a smaller net change of modules. Perhaps this was a consequence of secondary vegetation absence encircling the sites. In this case, there is little potential for invasion by other rhizomatous species.
Module reproduction was synchronized across sites. The greatest number of modules in each site coincides with the month following the first month (June) of the year with precipitation, and the lowest number of modules was observed during the months with less precipitation (October-December). In all plants water availability appears to favor growth and reproduction of other clonal species (Abrahamson 1980).
The general trend of genet survivorship curves among populations was similar. Survivorship across cohorts of the same age appeared to be more synchronized than across cohorts of different age but from the same site (Fig. 2). The mortality in Z. diploperennis genets, as in other rhizomatous species (Harper 1977, Lovett Doust 1981), declines with age.
The inverse relation between proportional genets mortality and age is likely to be due to asymmetric intraspecific competition (Crawley 1990), where older established individuals are competitively superior to their younger counterparts, as has been demonstrated for Solidago canadensis (Hartnett & Bazzaz 1985), Fagus crenata populations (Nakashizuka 1987 review of Crawley 1990) and Bouteloua gracilis populations (Aguilera & Lauenroth 1993). This pattern characterizes species where intraspecific competition appears to be responsible for the suppression of recruitment after the initial colonization (initial seedling recruitment, according to Eriksson (1993)).
As in the case of Ranunculus acris and R. bulbosus (Sarukhán & Harper 1973), the total number of genets per site in populations of Z. diploperennis exhibited a seasonal rhythm with peaks during spring time and was more evident in year two (Fig. 1, Fig. 2). The greatest genets mortality occurred during the season of greatest growth, i.e., the rainy seasons (July through September). This is similar to mortality patterns described for R. canadensis (Hartnett & Bazzaz 1985), Lolium perenne and other clonal grasses (Langer et al. 1964, Kays & Harper 1974). These patterns suggest that intraspecific competition may regulate genet mortality (Sarukhán & Harper 1973).
Growth active of rhizomatous perennial Z. diploperennis occurs immediately after the first rains following a dry season. As a consequence, we postulate that interspecific and intraspecific competition occurs principally during the rainy season, which coincides with the period when the maximum rates of genet mortality occur. Our results concur with the hypothesis offered by Hartnett & Bazzaz (1985), that clonal growth and genet survival of herbaceous clonal species (of the initial seedling recruitment type) are inversely related to time of recruitment. That is, long after initial colonization, populations of herbaceous clonal species will be formed mainly by the genets that became established initially, as hypothesized by Benz et al. (1990) (see also Kays & Harper 1974, Maddox et al. 1989, Eriksson 1993). This implies that reproduction from seeds is unimportant at these stages and might not be a viable alternative for achieving population increase.
The degradation of soils in the Sierra de Manantlán Biosphere Reserve is caused primarily by forest fires, slash and burn agriculture and cattle grazing (Pineda 1988, Jardel 1991, Sánchez-Velásquez & García-Moya 1993, Sánchez-Velásquez et al. 1996, Hernández 1998). Those areas with poor soil (Ultisol) or soils with similar physical characteristics could be rehabilitated by introducing Z. diploperennis. Such rehabilitation would achieve two distinct objectives, promote propagation of this rare endemic and reclaim areas that are susceptible to erosion and further degradation.
Acnowledgements
We are grateful to Oscar Cárdenas Hernández, Luis Manuel Martínez R., Eduardo Santana, Carlos Palomera and L. E. Goldman for reviewing the manuscript. Fieldwork was assisted by E. Cortez F., A. Cárdenas T., R. Lorente A., J. Rosales A., O. Cárdenas, R. Ramírez, F. Hernández and B. Figueroa. Victor Sánchez provided logistic support. Francisco Santana identified some plant species. This work was supported by grant D112-904275 from the Consejo Nacional de Ciencia y Tecnología-México, the University of Guadalajara and the World Wildlife Fund-US. This is a contribution to knowledge of plant ecology within Biosphere Reserves from MAB-UNESCO Program.
Resumen
Se estudió la demografía de Zea diploperennis en siete sitios de cultivo abandonados. La magnitud de las fluctuaciones demográficas fue más grande en las poblaciones de módulos. La dinámica de las poblaciones de genets siguió un ritmo estacional con un tamaño máximo de la población al inicio de la temporada de lluvias; las cohortes más jóvenes presentan la más alta mortalidad, i. e., Y=[ sin(-0.288x + 1.657)]2 , (r = 0.92, p < 0.01), donde es la proporción de la mortalidad anual y es la edad de la cohorte. La máxima tasa de mortalidad de genets ocurrió durante la temporada de lluvias cuando la densidad de la población fue más alta, cuando la competencia parece mayor. Hubo relaciones lineales entre la tasa de incremento de genets y el estado sucesional y el tipo de suelo. Aquellas áreas con suelos pobres degradados o suelos con similares características, pueden ser rehabilitados introduciendo Z. diploperennis, protegiéndola y combatiendo la degradación del suelo.
References
Abrahamson, W. G. 1980. Demography and vegetative reproduction, p. 89-106. In O. T. Solbrig (ed.). Demography and evolution in plant populations. Blackwell Scientific, Oxford.
Aguilera, M. O. & W. K. Lauenroth. 1993. Seedling establishment in adult neighborhoods- intraspecific constraints in the regeneration of the bunchgrass Bouteloua gracilis. J. Ecol. 81: 253-261. [ Links ]
Benz, B. F., L. R. Sánchez-Velásquez & F. Santana-Michel. 1990. Ecology and ethnobotany of Zea diploperennis: Preliminary investigations. Maydica 35: 85-98. [ Links ]
Boul, S. W., F. D. Hole & R. J. McCracken. 1993. Soil Genesis and Classification. Trillas, México, D. F. [ Links ]
Boyce, M. S. 1992. Population viability analysis. Ann. Rev. Ecol. Syst. 23: 481-506. [ Links ]
Brussard, P. F. 1991. The role of ecology in biological conservation. Ecol. Applic. 1: 6-12. [ Links ]
Crawley, M. J. 1990. The population dynamics of plants. Phil. Trans. Roy. Soc. Lond. 330: 125-140. [ Links ]
Eriksson, O. 1993. Dynamics of genets in clonal plants. TREE 8: 313-316. [ Links ]
Gilpin, M. E.& M. E. Soulé. 1986. Minimum viable populations: process of species extinction. p. 19-34. In M. E. Soulé (ed.). Conservation Biology. Sinauer, Massachusetts.
Halffter, G. 1988. El Concepto de Reserva de la Biosfera. p. 79-111. In P. Reyes-Castillo (ed.). El futuro del hombre en la naturaleza. Instituto de Ecología, Publicación No. 24. México. [ Links ]
Harper, J. L. 1977. Population Biology of Plants. Academic, London. [ Links ]
Hartnett, D. H. & F. A. Bazzaz. 1985. The genet and ramet population dynamics of Solidago canadensis in an abandoned field. J. Ecol. 73: 407-413. [ Links ]
Harvey, H. J. 1985. Population biology and the conservation of rare species. p. 111-123. In J. White (ed.). Studies on Plant Demography, 111-123. Academic, London.
Hernández, V. G. 1998. Efecto de la ganadería extensiva sobre la regeneración arbórea de los bosques subtropicales de montaña en la Estación Científica Las Joyas, Jalisco. Tesis de Biología, Universidad Veracruzana, Xalapa Veracruz, México. [ Links ]
Hoyt, E. 1988. Conserving the wild relative of crops. International Board of Plant Genetics Resources/World Conservation Union/World Wildlife Fund, Rome. [ Links ]
Huenneke, L. F. 1995. Involving academic scientists in conservation research: perspectives of a plant ecologist. Ecol. Appl. 5: 209-214. [ Links ]
Hutchings, M. & I. K. Bradbury. 1986. Ecological perspectives on clonal perennial herbs. BioScience 36: 178-182. [ Links ]
Iltis, H. H., J. F. Doebley, R. Guzmán & B. Pazy. 1979. Zea diploperennis (Gramineae): A new teosinte from México. Science 203: 186-188.
Jardel, P. E. J. 1991. Perturbaciones naturales y antrópicas y sus influencia en la dinámica sucesional de los bosques de Las Joyas, Sierra de Manantlán. Tiempos de Ciencia 22: 9-26 [ Links ]
Jardel, P. E. J. 1992. Estrategia para la conservación de la Reserva de la Biosfera Sierra de Manantlán. Universidad de Guadalajara, Guadalajara Jalisco, México.
Kays, S. & J. L. Harper. 1974. The relation of plant and tiller density in a grass sward. J. Ecol. 62: 97-105. [ Links ]
Langer, R. H., S. M. Ryle & O. R. Jewis. 1964. The changing plant and tiller populations of timothy and meadow fescue swards. I. Plant survival and the pattern of tillering. J. Ecol. 1: 197-208. [ Links ]
Lesica, P. 1992. Autoecology of the endangered plant Howellia aquiatilis; implications for management and reserve design. Ecol. Appl. 2: 411-421. [ Links ]
Lorente-Adame, R. G. & L. R. Sánchez-Velásquez. 1996. Dinámica estacional del banco de frutos del teocintle Zea diploperennis (Gramineae). Biotropica 28: 267-272. [ Links ]
Lovett Doust, L. 1981. Population dynamics and local specialization in a clonal perennial (Ranunculus repens): I. The dynamics of ramet in contrasting habitats. J. Ecol. 69: 743-755. [ Links ]
Maddox, G. D., R. E. Cook, P. H. Wimberger & S. Gardescu. 1989. Clone structure in four Solidago altissima (Asteraceae) populations: rhizome connections within genotypes. Amer. J. Bot. 76: 318-326.
Olmsted, Y. & E. R. Alvarez-Buylla. 1995. Sustainable harvesting of tropical trees: demography and matrix models of two palm species in Mexico. Ecol. Appl. 5: 484-500. [ Links ]
Owen, W. R. & R. Rosentreter. 1992. Monitoring rare perennial plants: techniques for demographic studies. Nat. Areas. J. 12: 32-38. [ Links ]
Pérez, S. B. 1991. Estudio fitosociológico de Zea diploperennis en la Estación Científica Las Joyas, Sierra de Manantlán, Jalisco, México. Tesis de Biología, Universidad de Guadalajara, Guadalajara, Jalisco, México. [ Links ]
Pineda-López, Ma. del R. 1988. Efecto de las perturbaciones sobre la estructura y dinámica de los bosques templados de Las Joyas, Sierra de Manantlán. Tesis de Maestría, Instituto Nacional de Investigaciones sobre Recursos Bióticos, Xalapa, Veracruz, México. [ Links ]
Rabinowitz, D., S. Cairs & T. Dillon. 1986. Seven forms of rarity and their frequency in the flora of the British Island. p. 182-204. In M. E. Soulé (ed.). Conservation Biology. Sinauer, Sunderland, Massachusetts. [ Links ]
Sánchez-Velásquez, L. R. 1991. Zea diploperennis: mejoramiento genético del maíz, ecología y la conservación de recursos naturales. Tiempos de Ciencia 24: 1-8. [ Links ]
Sánchez-Velásquez, L. R., Solís, M. A., E. Cortéz, R. G. Jiménez-G, A. Cárdenas & M. P. Rosales. 1991. Efecto del cultivo tradicional del maíz sobre el crecimiento y desarrollo de Zea diploperennis. Biotam 3: 51-58.
Sánchez-Velásquez, L. R. & García-Moya. 1993. Sucesión en los bosques mesófilo de montaña y de Pinus de la Sierra de Manantlán, Jalisco, México. Agrociencia 3: 7-26. [ Links ]
Sánchez-Velásquez, L. R. & R. G. Lorente-Adame. 1993. Sobrevivencia de frutos de Zea diploperennis en diferentes hábitats. Biotam 5: 29-34. [ Links ]
Sánchez-Velásquez, L. R., J. J. Rosales-Adame, Ma. del R. Pineda-López & Ma. de los A. Saldaña-Acosta. 1996. Descripción cuantitativa de los bosques en la estación científica Las Joyas de la Reserva de la Biosfera Sierra de Manantlán. Bol. Inst. Bot. U. de G. México 4: 79-155. [ Links ]
Sarukhán, J. & J. L. Harper. 1973. Studies on plant demography: Ranunculus repents L., R. bulbosus L. and R. acris L. I. Population flux and survivorship. J. Ecol. 61: 675-716. [ Links ]
SAS. 1988. SAS Stat user's guide for personal computers, version 6.03. SAS Institute, Cary, North Carolina. [ Links ]
Seaman, J. W. Jr., S. C. Walls, S. E. Wise & R. G. Jaeger. 1994. Caveat emptor: rank transform methods and Interaction. TREE 9: 261-263. [ Links ]
Tear, T. H., J. M. Scott, P. H. Hayward & B. Griffith. 1995. Recovery plans and the endangered species act: are criticisms supported by data? Cons. Biol. 9: 182-195. [ Links ]
Watson, L. E., G. E. Uno, N. A. McCarty & B. Kornkuen. 1994. Conservation biology of a rare plant species, Eriocaulon kornickianum (Eriocaulaceae). Am. J. Bot. 81: 980-986.
Wilkes, H. G. 1994. El teosinte en México como modelo para la conservación in situ: un reto. p. 257-270. In B. F. Benz (ed.). Biología, ecología y conservación del género Zea, Universidad de Guadalajara, México.
1 Facultad de Ciencias, Universidad Nacional Autónoma de México, 04510, México D.F. México.
2 Instituto Manantlán de Ecología y Conservación de la Biodiversidad, Centro Universitario de la Costa Sur,
Universidad de Guadalajara, Independencia Nacional No. 151. Ap. Postal No. 64, Autlán, Jal., 48900, México; Fax + 338 11425;lsanchez@cucsur.udg.mx
3 Biology Department, Texas Wesleyan University, Fort Worth, TX 76105; e-mail: benzb@txwes.edu














