Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.49 n.1 San José Mar. 2001
Stephan Imhof 1
Recibido 5-IV-2000. Corregido 17-VIII-2000. Aceptado 19-IX-2000
Abstract
Plants of Dictyostega orobanchoides arise from about 1 mm thick rhizomes, which are densely covered by sessile, imbricate, peltate scale leaves. The resulting interfoliar spaces are inhabited by fungal hyphae up to 6 µm thick, often developing vesicle-like bladders. The fungus also colonizes the tissue of the scale leaves, inter- as well as intracellularly, forming vesicles but no arbuscules, and it even penetrates the vascular bundles of the leaves. The rhizome itself does not become infected. The 200 µm thick roots emerge from the rhizome and have a 2-layered cortex and voluminous rhizodermis, which both are delicate and often disrupted or missing. In contrast, the strongly reinforced, tertiary endodermis and the central cylinder are durable and have a considerable tensile strength. Although the roots grow through the hyphal masses in the interfoliar spaces when emerging from the rhizome, they only become infected from the rhizosphere. A collar of rhizomogenous tissue hinders the interfoliar hyphae from direct contact to the roots. Only within the rhizodermis, the mycorrhizal fungus builds coils of heteromorphic hyphae, arbuscule-like structures, and vesicles. Hence, the mycorrhiza in D. orobanchoides is assigned to the arbuscular mycorrhiza. It is hypothezised, that the ephemeral mycorrhizal tissue combined with the durable vascular system of the roots is a strategy to avoid the high costs of protecting the large rhizodermal surface area. The rhizomogenous collar is explained as an extra protection to the tender, young roots, when emerging from the rhizome. The necessity to include other subterranean plant organs along with the roots in future mycorrhizal studies is emphasized.
Key words: Dictyostega, Burmanniaceae, root structures, rhizome structures, arbuscular mycorrhiza, myco-heterotrophy.
In April 1837 John Miers collected close to Rio de Janeiro a curious, achlorophyllous, almost leafless plant, with a slender, rarely branched stem, bearing a terminal bifurcate, cincinnous (scorpioid cyme) inflorescence (Miers 1841). This specimen is mentioned in an unpublished manuscript by Miers as Dictyostegia orobanchoides, but was first published as Apteria orobanchoides (Burmanniaceae) by Hooker (1840). Miers transferred it (1840, 1841, erroneously as orobanchioides) to Dictyostega. Since then, Dictyostega species became mentioned in several taxonomic reports under various synonyms (Seubert 1847, Bentham 1855, Karsten 1858, Engler 1889, Warming 1901, Jonker 1938) until Maas (1981) merged them into one species (Dictyostega orobanchoides (Hook.) Miers) comprising three subspecies (full descriptions in Maas et al. 1986). They occur throughout the Neotropics from Mexico to Bolivia and southeast Brazil, from sea level to 2600 m (Maas et al. 1986). Anatomical investigations were only made by Johow (1889) and Rübsamen (1986), who both concentrated on embryology. However, achlorophyllous plants require an explanation of how they provide for their carbon. The answer to this question should be found in the subterranean organs, and be either of parasitic (Weber 1993) or of myco-heterotrophic (Leake 1994) nature. Only Johow (1889) gave a short description of D. orobanchoides root anatomy, but did not mention the root fungus. Despite the frequent demands for more investigation of myco-heterotrophic plants, particularly on species not in Orchidaceae and Monotropaceae (Hadley 1986, Leake 1994, Smith and Read 1997), we are only gradually learning more on that subject. In a series of papers on achlorophyllous Gentianaceae (Imhof et al. 1994, Imhof and Weber 1997, Imhof 1997, Imhof 1999a), Triuridaceae (Imhof 1998a) and Burmanniaceae (Imhof 1999b, Imhof 1999c) it became evident that the mycorrhizae of these myco-heterotrophic plants are highly diverse, but have many features in common, suggesting an arbuscular mycorrhiza. The combination of an obligate biotrophic fungus and an achlorophyllous host seems to be contradictory, but has been discussed to be conceivable (Imhof and Weber 1997). This article aims to elucidate the mycorrhiza of Dictyostega orobanchoides, the first species in this series of papers to have a typical rhizome.
Materials and methods
About 40 plants of Dictyostega orobanchoides (Hook.) Miers ssp. parviflora (Bentham) Snelders and Maas were collected in February 1995 in a rainforest in Estado Bolívar/Venezuela, coordinates N05°5744/W61°3014, ca. 700 m above sea level (Herbarium of Stephan Imhof No. 173). Representative duplicates are deposited in the Herbarium of the Universidad Central, Caracas.
The plants were carefully extracted from the subtrate, and either preserved as herbarium specimen or immediately fixed in FPA (37%-Formalin : Propionic acid : 50%-Ethanol at 0.5 : 0.5 : 9) and later transferred into 70%-Ethanol for storage. After dehydration in an ascending ethanol-series, rhizomes and root samples of about 5 randomly selected specimens were embedded in UnicrylTM (British Biocell Int.). The hardened, embedded objects were cut in 2-5 µm sections (Reichert/Jung Supercut 2065), stained with 1% Toluidine Blue O-Solution after Krause (1927), and preserved in Corbit-Balsam as permanent slides. Suberin and cutin were detected in freehand sections of fixed material by using a saturated solution of Sudan IV in 70% ethanol, differentiated with glycerol. Starch was demonstrated using an iodine-potassium iodide solution (Johansen 1940). Lignin was also tested in freehand sections with Phloroglucinol/HCl (Jensen 1962).
Samples for scanning electron microscopy were dehydrated through ethylene glycol monoethyl ether, washed twice in acetone, dried by the 'critical point method' (Anderson 1951), and coated with gold in a Balzers Union Sputter Coater.
Results
Dictyostega orobanchoides ssp. parviflora grows superficially in an array of loose substrates from sand to organic litter, often on decaying wood. At the collection site it was by far the most frequent achlorophyllous plant species, tending to occur in clusters.
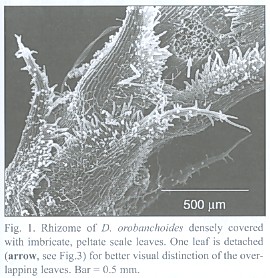
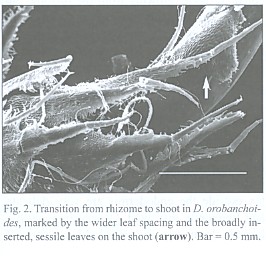
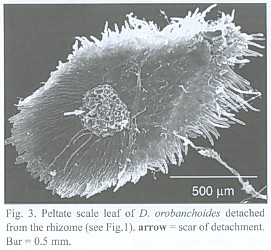
The subterranean part consists of an up to 1 mm thick rhizome, found to be up to 4 cm long, densely covered with imbricate, narrowly ovate, long acuminate scale leaves that are rounded at the base. The scale leaves are fringed with cilia and sometimes bear small hairs on the abaxial side (morphological lower side, Fig. 1). The rhizomes directly transform into the epiterraneous stem, a transition which is easily characterized by the wider scale leaf spacing on the stem (Fig. 2), and a noteworthy change in the mode of scale leaf insertion: in contrast to the also sessile but rather broadly inserted scale leaves of the stem (Fig. 2), the rhizome scale leaves are peltate (Fig. 3). Rhizomes as well as stems do branch occasionally.
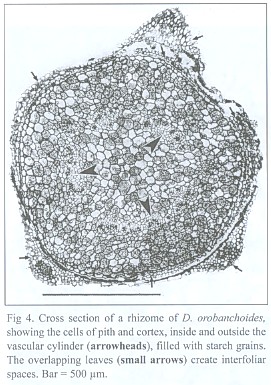
The dictyostelar vascular cylinder of the rhizome consists of sometimes leptocentric, but mostly indistinctly built, bundles. The bundles show strong reaction to phloroglucinol/HCL, proving that they are lignified. In contrast to the gaps created by the leaf traces, roots do not leave gaps in the vascular cylinder. The pith as well as the cortex is made up of isodiametric parenchymatic cells containing starch grains (Fig. 4). The cells of the epidermis are smaller and have a thick external cutin layer.
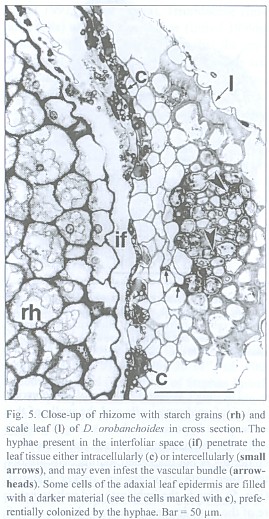
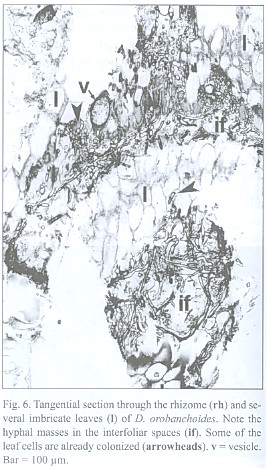
The inner tangential walls of the abaxial scale leaf epidermis cells and the walls of the subsequent hypodermis show strong, lignified cell wall depositions. The adaxial scale leaf epidermis is not thickened, but its cells are often filled with an unknown, darker material (Fig. 5). Due to the imbricate and peltate nature of the scale leaves, up to three layers of leaf blades may cover the rhizome (see Fig. 4). Accordingly, up to three interspaces can be present between rhizome and the covering scale leaves. These spaces contain masses of intermingled, aseptate fungal hyphae, up to 6 µm thick, frequently producing vesicle-like bladders (Fig. 6). Moreover, those hyphae penetrate the adaxial leaf epidermis, preferentially colonizing the cells with the darker content. They also can be seen deeper in the leaf tissue, either intercellularly or building intracellular coils of thick hyphae and vesicles, and may even colonize the vascular bundle (see Fig. 5). Arbuscules were not found in leaf tissue, and neither was degenerated fungal material as found in the roots (see below). The fungus does not penetrate the rhizome epidermis.
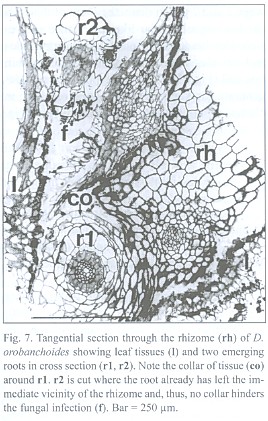
Rhizome-borne roots breaking through the leaf layers necessarily have to grow through the hyphal layers in the interfoliar spaces too, but an infection of the roots there was never found. A collar of tissue, growing from the rhizome but lacking starch grains, envelopes the roots at their base, seemingly protecting them from such a direct infection (Fig. 7). Hence, the roots must become colonized from the rhizosphere, not from the interfoliar spaces.
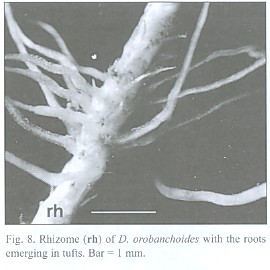
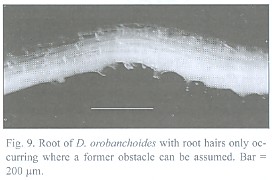
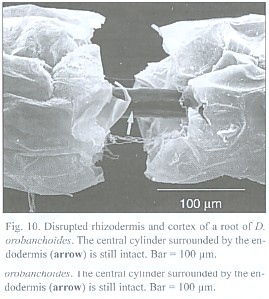
The about 200 µm thick and up to 5 cm long roots tend to emerge in tufts from the rhizome (Fig. 8) and can branch repeatedly. Short root hairs were only observed where the root has grown around an obstacle, as suggested by its curved shape (Fig. 9). Rhizodermis and cortex outside the endodermis are very delicate and frequently disrupted. Observations with a large scale microscope revealed large parts of the root bare of cortex and rhizodermis (Fig. 10). The remaining endodermis and central cylinder prove to have considerable tensile strength when handled with tweezers, considering the otherwise tender character of the plant.
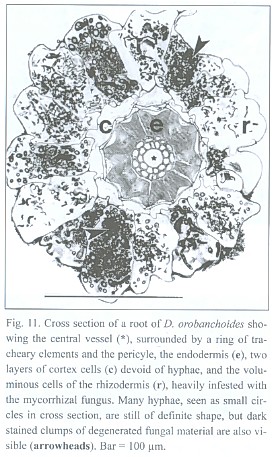
The lignified central cylinder of the roots consists of a single, relatively voluminous vessel, next surrounded by a ring of smaller tracheary elements and subsequently by a cell layer often containing cytoplasmic content and missing the helical or reticulate thickenings of the tracheary cells underneath. However, the arrangement of the tissue surrounding the central trachea may be blurred. The endodermis cells have exceptionally strong, lignified, u-shaped thickenings, leaving a small cell lumen only. Two layers of small cells with no visible content follow outside the endodermis. The most spacious part of the root is occupied by the rhizodermis (Fig. 11).
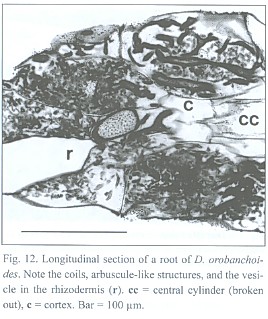
Penetrations of the rhizodermis by the mycorrhizal fungus show, if at all, only small appressoria. The exclusively intracellular hyphae are restricted to the rhizodermis cells only (see Fig. 11), building rather heteromorphic coils. The frequently branched hyphae can range between 2 and 7 µm in diameter. Parts of the coils can have an arbuscular-like appearance and vesicle-like bladders do occur. Such arbuscules and vesicles are often found together within one cell (Fig. 12). Thinner hyphae seem to degenerate quicker than thicker ones, resulting in clumps of amorphous fungal material (see Fig. 11 and 12).
Discussion
The root and rhizome structures of Burmanniaceae have been classified by Johow (1889), Goebel and Süssenguth (1924) and Maas et al. (1986). They chiefly agree on three main types:
1. Tubers in combination with filiform roots in some Thismia species (Poulsen 1890, Warming 1901, Goebel and Süssenguth 1924).
2. Thick, succulent, vermiform roots occuring in other Thismia species (e.g. Groom 1895, Campbell 1968, Pfeiffer 1914, Bernard and Ernst 1910) as well as in Burmannia tenella (Imhof 1999b).
3. Only slightly swollen, foliose rhizomes in combination with long, filiform roots observed in most Burmanniaceae (Apteria, Burmannia, Cymbocarpa, Dictyostega, Gymnosiphon, Hexapterella, Marthella, Miersiella).
Intermediate forms occur as well, since Maas et al. (1986) reported the occurrence of fusiform tubers at the end of roots of Burmannia alba and B. grandiflora, and of a cylindric, tuberous rhizome of Campylosiphon purpurascens.
Despite their unequal subterranean structures, these species, as far as investigated, often share many mycorrhizal features, concerning size and appearance of hyphae and vesicles, as well as the intracellular mode of infestation. This is particulary the case in the mycorrhiza in Dictyostega orobanchoides, which strongly resembles the one in Burmannia tenella. But compared to B. tenella, the roots of which have a large mycorrhizal cortex parenchyma (Imhof 1999b), D. orobanchoides with its single mycorrhizal root layer needs longer and/or more roots to lodge a similar amount of the nutrimental mycorrhizal tissue (see Imhof 1999b). A reinforcement of the accordingly much larger rhizodermal surface area would be quite costly, and in fact, the rhizodermis of D. orobanchoides, after all hosting the nourishing fungus, is not visibly protected, very delicate and frequently disrupted. But this strategy of having an ephemeral mycorrhizal tissue, essentially depends on a reliable transporting system, transferring all the carbohydrates, water and nutrients acquired by the mycorrhizal rhizodermis to the rhizome for storage, independent of wether the unprotected rhizodermis and cortex are still present or not. Therefore, the great tensile strength of the endodermis/central cylinder complex is of eminent ecological importance. In compliance with its function to convey the matter from root to shoot rather than the opposite direction, the central cylinder of D. orobanchoides mainly consists of tracheary elements. The only remaining layer, surrounding the tracheary cells, can either be pericycle or phloem, one of which must have been abolished. Because phloem tissue, regarded to supply tissues with carbohydrates, seems senseless where the carbohydrates are provided by the mycorrhizal fungus close by, and because the roots of D. orobanchoides do branch frequently, requiring a meristematic tissue, the concerned layer must be pericyle. Confirmatory, Hirce and Finocchio (1972) also recorded reduced phloem tissue for the likewise myco-heterotrophic Monotropa uniflora.
Especially achlorophyllous, mycotrophic plants, which essentially rely on their mycorrhiza, cannot afford to waste or overuse their mycorrhizal fungus (Imhof 1998b), and, correspondingly, mycorrhizal structures were found in myco-heterotrophic plants allowing a sustained benefit from the endophyte for the plant (Imhof 1997, Imhof 1998a, Imhof 1999c). Likewise, it would appear as an elegant and sensible strategy, if young roots emerging from the rhizome of D. orobanchoides were supplied with the mycorrhizal fungus simply by growing through the layers of hyphae present in the interfoliar spaces. But a fungal infection, even a mycorrhizal one, of the immature root tissue, might be hazardous, and malfunction of especially the central cylinder (see above) in this situation would be fatal to the whole absorbing system. Hence, the extra protection by the rhizomogenous collar might be an essential feature. The necessary mycorrhizal infection must happen from the rhizosphere, where the root hairs, probably selectively developed where roots contact some adequate substrate, might help in the process of fungus acquisition. This phenomenon has already been shown in Voyria aphylla (Imhof 1999a).
Like in Burmannia tenella, the vesicles and arbuscule-like structures suggest an arbuscular mycorrhiza for D. orobanchoides. In B. tenella (Imhof 1999b), it was speculated that the earlier digestion of thinner hyphae while keeping the thick hyphae functional for longer might serve for some hyphal maintenance within the roots. This could hold for D. orobanchoides too. But the extensive interfoliar spaces on its rhizome, provided by the imbricate and peltate scale leaves, inhabited by fungal hyphae, may represent an additional, more structural adaptation in order to facilitate fungal conditions. Since, moreover, the leaf tissue becomes colonized without signs of fungal degeneration, in contrast to the hyphae in the rhizodermis, the interfoliar spaces seem even more to be a favourable place for the fungus. The darker material in cells of the adaxial epidermis might play some nourishing or attracting role in this regard. Provided the root fungus and the fungus in the interfoliar spaces are identical, which is not proved but highly probable, the described mycorrrhizal pattern can once more be interpreted as a sustained or at least prolonged use of mycorrhiza: the rhizome with scale leaves may serve for fungal attraction and retention, resulting in a fungal rich rhizomosphere. This promotes the infection of the roots sent therein, and in the rhizodermis the hyphae become digested. Eventually, the digested matter is transported to the rhizome for storage through the durable central cylinder/endodermis complex. Interpretation of anatomical or morphological structures in terms of function inevitably stays speculative. This, however, must not deter us from bringing up hypotheses about possible purposes of structures, always led by the principle of the least complicated explanation. Physiological investigations are required to test the raised hyphotheses, if necessary under developing suitable techniques.
There are only few other reports of mycorrhizal fungi in leaf tissues (Taber and Trappe 1982, Stasz and Sakai 1984, Mago et al. 1993, on Zingiberaceae), also describing seemingly beneficial associations for the fungi in rhizome leaves, notwithstanding their entirely different structures in the roots. These data extend our knowledge on the diversity of mycorrhizas. Hence, it is strongly recommended to include any subterranean, and even epiterranean (see Imhof 1999c), tissues in our search for mycorrhizal structures, something which has been largely neglected in the past.
Acknowledgements
I thank Hiltje and Paul Maas, Utrecht, for their hospitality during my stay in Utrecht, their help in identifying myco-heterotrophic plants, and for many further hints and suggestions. I also thank Andrew Smith, Adelaide, for his suggestions on the manuscript. In particular, I am grateful to my adviser Hans Christian Weber.
Resumen
Plants of Dictyostega orobanchoides arise from about 1 mm thick rhizomes, which are densely covered by sessile, imbricate, peltate scale leaves. The resulting interfoliar spaces are inhabited by fungal hyphae up to 6 µm thick, often developing vesicle-like bladders. The fungus also colonizes the tissue of the scale leaves, inter- as well as intracellularly, forming vesicles but no arbuscules, and it even penetrates the vascular bundles of the leaves. The rhizome itself does not become infected. The 200 µm thick roots emerge from the rhizome and have a 2-layered cortex and voluminous rhizodermis, which both are delicate and often disrupted or missing. In contrast, the strongly reinforced, tertiary endodermis and the central cylinder are durable and have a considerable tensile strength. Although the roots grow through the hyphal masses in the interfoliar spaces when emerging from the rhizome, they only become infected from the rhizosphere. A collar of rhizomogenous tissue hinders the interfoliar hyphae from direct contact to the roots. Only within the rhizodermis, the mycorrhizal fungus builds coils of heteromorphic hyphae, arbuscule-like structures, and vesicles. Hence, the mycorrhiza in D. orobanchoides is assigned to the arbuscular mycorrhiza. It is hypothezised, that the ephemeral mycorrhizal tissue combined with the durable vascular system of the roots is a strategy to avoid the high costs of protecting the large rhizodermal surface area. The rhizomogenous collar is explained as an extra protection to the tender, young roots, when emerging from the rhizome. The necessity to include other subterranean plant organs along with the roots in future mycorrhizal studies is emphasized.
Referencias
Anderson, T. F. 1951. Techniques for the preservation of three-dimensional structure in preparing specimens for the electron microscope. Trans. New York Acad. Sci., Ser. II 12: 130-134.
Bentham, G. 1855. On the South American Triurideae and leafless Burmanniaceae from the collections of Mr. Spruce. Hooker's J. Bot. Kew Gard. Misc. 7: 8-17. [ Links ]
Bernard, C. & A. Ernst. 1910. Beiträge zur Kenntnis der Saprophyten Javas. II. Äussere und innere Morphologie von Thismia javanica J. J. S. Ann. Jard. Bot. Buitenzorg 23: 36-46. [ Links ]
Campbell, E. O. 1968. An investigation of Thismia rodwayi F. Muell. and its associated fungus. Trans. Roy. Soc. New Zealand 3: 209-219. [ Links ]
Engler, A. 1889. Burmanniaceae, p. 44-51. In: Engler A, Prantl K (ed) Die Natürlichen Pflanzenfamilien. Engelmann, Leipzig. [ Links ]
Goebel, K. & K. Süssenguth. 1924. Beiträge zur Kenntnis der südamerikanischen Burmanniaceen. Flora 117: 55-90. [ Links ]
Groom, P. 1895. On Thismia aseroe (Beccari) and its mycorhiza. Ann. Bot. 9: 327-361. [ Links ]
Hadley, G. 1986. Mycorrhizas of heterotrophic plants, p. 815-819. In V. Gianinazzi-Pearson & S. Gianinazzi (eds.). Physiological and genetical aspects of mycorrhizae. Proc. 1st European Symp. Mycorrhizae 1-5 July, Dijon. INRA, Paris.
Hirce, E. G. & A. F. Finocchio. 1972. Stem and root anatomy of Monotropa uniflora. Bull.Torrey Bot. Club 99: 89-94. [ Links ]
Hooker, W. J. 1840. Apteria orobanchoides. Icon. Pl. 3: t. 254. [ Links ]
Imhof, S., H. C. Weber & L. D. Gómez. 1994. Ein Beitrag zur Biologie von Voyria tenella Hook. und Voyria truncata (Standley) Standley and Steyermark (Gentianaceae). Beitr. Biol. Pflanzen 68: 113-123. [ Links ]
Imhof, S. & H. C. Weber. 1997. Root anatomy and mycotrophy (AM) of the achlorophyllous Voyria truncata (Standley) Standley and Steyermark (Gentianaceae). Bot. Acta 110: 127-134.
Imhof, S. 1997. Root anatomy and mycotrophy of the achlorophyllous Voyria tenella Hooker (Gentianaceae). Bot. Acta 110: 298-305. [ Links ]
Imhof, S. 1998a. Subterranean structures and mycotrophy of the achlorophyllous Triuris hyalina Miers (Triuridaceae). Can. J. Bot. 76: 2011-2019. [ Links ]
Imhof, S. 1998b. Strategies for sustainable use of mycorrhiza in myco-heterotrophic plants. Swedish University of Agricultural Science, Second International Conference on Mycorrhiza, July 5-10 1998. Uppsala, Sweden, 86 p.
Imhof, S. 1999a. Root morphology, anatomy and mycotrophy of the achlorophyllous Voyria aphylla (Jacq.) Pers. (Gentianaceae). Mycorrhiza 9: 33-39. [ Links ]
Imhof, S. 1999b. Subterranean structures and mycorrhiza of the achlorophyllous Burmannia tenella Benth. (Burmanniaceae). Can. J. Bot. 77: 637-643.
Imhof, S. 1999c. Anatomy and mycotrophy of the achlorophyllous Afrothismia winkleri (Engl.) Schltr. (Burmanniaceae). New Phytol. 144: 533-540. [ Links ]
Jensen, W. A. 1962. Botanical Histochemistry. Freeman, San Francisco, 408 p. [ Links ]
Johansen, D. A. 1940. Plant Microtechnique. McGraw-Hill, New York, 523 p. [ Links ]
Johow, F. 1889. Die chlorophyllfreien Humuspflanzen nach ihren biologischen und anatomisch-entwicklungsgeschichtlichen Verhältnissen. Jahrb. Wiss. Bot. 20: 475-524.
Jonker, F. P. 1938. A monograph of the Burmanniaceae. Meded. Bot. Mus. Herb. Rijks Univ. Utrecht 51: 1-279. [ Links ]
Karsten, H. 1858. Über die Stellung einiger Familien parasitischer Pflanzen im natürlichen System. Nov. Actorum Acad. Caes. Leop.-Carol. Nat. Cur. 26: 885-924. [ Links ]
Krause, R. 1927. Enzyklopädie der Mikroskopischen Technik. Urban und Schwarzenberg, Berlin, 2444 p. [ Links ]
Leake, J. J. 1994. Tansley Review No. 69. The biology of myco-heterotrophic ('saprophytic') plants. New Phytol. 127: 171-216. [ Links ]
Maas, P. J. M. 1981. Notes on new world saprophytes II. Acta Bot. Neerl. 30: 139-149. [ Links ]
Maas, P. J. M., H. Maas-van de Kamer, J. v. Benthem, H. C. M. Snelders & T. Rübsamen. 1986. Burmanniaceae. Flora Neotropica Monograph 42: 1-189.
Mago, P., P. Pasricha & K. G. Mukerji. 1993. Survey of vesicular-arbuscular mycorrhizal fungi in scale leaves of modified stems. Phytomorph. 43: 59-70. [ Links ]
Miers, J. 1840. On some new Brazilian plants allied to the natural order Burmanniaceae. Proc. Linn. Soc. London 1: 60-63. [ Links ]
Miers, J. 1841. On some new Brazilian plants allied to the natural order Burmanniaceae. Trans. Linn. Soc. London 18: 535-556, t 37-38. [ Links ]
Pfeiffer, N. E. 1914. Morphology of Thismia americana. Bot. Gaz. 57: 122-135. [ Links ]
Poulsen, V. A. 1890. Thismia Glaziovii nov. sp.. Overs. Kongel. Danske Vidensk. Selesk. Forh. Medlemmers Arbeider 1890: 18-38.
Rübsamen, T. 1986. Morphologische, embryologische und systematische Untersuchungen an Burmanniaceae und Corsiaceae (Mit Ausblick auf die Orchidaceae-Apostasioideae). Diss. Bot. 92: 1-310.
Seubert, M. 1847. Burmanniaceae, p. 53-60. In: Martius CFPv (ed) Flora Brasil. 3,1. [ Links ]
Smith, S. E. & D. J. Read. 1997. Mycorrhizal symbiosis. Acadamic, San Diego, California, 605 p. [ Links ]
Stasz, T. E. & W. S. Sakai. 1984. Vesicular-arbuscular mycorrhizal fungi in scale-like leaves of Zingiberaceae. Mycologia 76: 754-756. [ Links ]
Taber, R. A. & J. M. Trappe. 1982. Vesicular-arbuscular mycorrhizae in rhizomes, scale leaves and xylem of ginger. Mycologia 74: 156-161. [ Links ]
Warming, E. 1901. Sur quelques Burmanniaceés recueillies au Brésil par le Dr. A. Glaziou. Oversigt over Kgl. Danske Vidensk. Selsk. Forhand. 1901: 173-188.
Weber, H. C. 1993. Parasitismus von Blütenpflanzen. Wissenschaftl. Buchges., Darmstadt, 168 p. [ Links ]
1 Morphologie und Systematik der Höheren Pflanzen, Fachbereich Biologie, Philipps-Universität, 35032 Marburg, Germany
Tel.: 06421-2825185; Fax: 06421-2822057. E-Mail: imhof@mailer.uni-marburg.de














