Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.49 n.1 San José Mar. 2001
Recibido 24-IX-1999. Corregido 19-VII-2000. Aceptado 19-IX-2000
Abstract
Tropical river sardine, Caquetaia kraussii, captured from La Aguá lagoon (Sucre State, Venezuela) were acclimatized for four weeks at 22, 24, 30 and 32ºC and at 0, 5, 10, 15 and 17 salinity. To evaluate effects of thermal response to acclimatization level, the fish were transferred suddenly from lower temperatures (22 and 24ºC) to higher ones (32 and 30ºC) respectively. Then thermal resistance time was measured at the lethal temperature of 40.9ºC for 30 days. We considered that acclimatization process completed when resistance time was stabilized at the new temperature regime. For the saline effect, the concentrations of sodium and potassium were measured in the tissues at each treatment: gills, white muscle, gut and heart. The results showed that thermal tolerance increased rapidly in 3 h with a 6ºC rise in temperature (from 24 to 30ºC) and in 24 h with a 10ºC rise (22 to 32ºC). With decreasing temperatures, the acclimatization level reached its lowest in 11 days with a 6ºC decreases (from 30 to 24ºC) and in 14 days with a 10ºC decrease (32 to 22ºC). Caquetaia kraussii regulates as much sodium as potassium in gills and white muscle tissues at all salinity levels tested; however, gut and heart tissues showed significantly different regulations among salinities examined.
Keywords: Thermal acclimatization, salinity, electrolytic concentration, Caquetaia kraussii
Temperature and salinity are the abiotic factors that have the strongest influence on organisms because they involve functional and structural alterations through changes in the osmotic concentration, promoting modification of behavior of a species inside an ecosystem. Also, salinity may establish differences in physiological effects in the embryonic development depending on other environmental factors, particularly temperature (Bennett & Judd 1992, Battaglane & Talbot 1993, Chung & Strawn 1994).
Knowledge of these two factors is very useful in studying tropical organisms in coastal lagoons, temporary shallow water lagoons, lakes and estuaries, since they are exposed to temperature fluctuations during the day and night and to salinity changes due to evaporation during dry periods or dilution during rainy seasons (Segnini et al. 1993, Segnini de Bravo & Chung 1997).
Fish living in a freshwater environment have higher rates of glomerular filtration and renal excretion of water and salts than those living in a seawater environment, because there is a hypoosmotic relation to their environment. Therefore, they have less electrolytic concentration than the seawater variety, absorbing Na+, K+ and Cl- ions, mainly in the digestive tract and desalinizing the water at gill levels. In euryhaline fish, the glomerular filtration rate and urine flow changes in the direction of ion concentrations, which means that it passes from a lower saline ion concentration to a higher one and vice versa. Although the nature of this mechanism is not known, it may be assumed that these changes are promoted in renal functions; furthermore they may participate in circulatory and hormonal functions (Canahan et al. 1986, Salman & Eddy 1988).
In spite of the physiological importance and ecological consequences of temperature and salinity on the stenohaline and euryhaline organisms, the available information is scarce. The aim of this work is to study the influence of acclimatization temperature and salinity in river sardine Caquetaia, which is a great food source for its excellent flavour, few bony axial skeleton, low fat and high protein contents.
Materials and methods
The organisms were captured in La Aguá lagoon, near Chiguana, Estado Sucre, Venezuela (10°30´N and 63°41´W) and transferred to the laboratory in insulated containers to avoid abrupt changes of temperature.
Later on, groups of 200 fish of 13.0 ± 1.5 mm in standard length (L) were acclimatized to temperatures of 22, 24, 30 and 32°C, respectively (the temperature range in La Aguá lagoon during the year). For their acclimatization, aquaria with a 300 l capacity, a thermal controller, a heater and a contact thermometer to regulate water temperature with a variation of ±0.01°C, were used. Acclimatization rates of two temperature changes were studied: a difference of 6ºC (24 to 30ºC) and of 10°C (22 to 32°C). Before the experiment and after transfer up to 30 days, 10 fish, taken at random with replica, were exposed to the lethal temperature of 40.9ºC to measure their thermal resistance time. We considered that acclimatization level had been completed when the survival time of the organisms progressively stabilized at a higher or lower temperature.
To study the incipient lethal salinity, three groups of 20 fish (10.0 ± 3.3 mm L), acclimatized at 23ºC with their respective replica and control were placed in freshwater aquaria. Then salinity was increased daily by 2 up to 15. After this, the increment was 1 per day, because it is known that in Colombia this species can be found in 14 salinity water (Hurtado 1975).
To obtain the Na+ and K+ ion concentrations, groups of 10 fish and their replica (80 ± 10 mm L) were acclimatized to five salinities (0, 5, 10, 15 and 17) at a constant temperature of 23°C. Each experimental salinity was reached in the way described previously. After a 4 week saline acclimatization, the fish were sacrificed by beheading; heart, muscular, gill and gastric tissue samples were taken, using a scalpel, taking care of always cutting in the same place and approximately the same amount (complete heart, complete gut, 4 thin sheets of gills and segments of white muscle). They were weighed immediately after the dissection, placed in plastic bags and then frozen at -17°C until the ion concentration was determined. The tissues were placed separately (muscle, heart, gill and gut) in a Kjeldahl flask. Nitric acid (3ml, 50%) was added and organic matter destroyed by being boiled for approximately 5-8 min. After this, a 5 ml liquid sample with the intratissular ions was obtained and placed in assay tubes to determinate ionic concentrations later on. The determination of ionic concentration of sodium and potassium was carried out in a direct reading by flame photometer. The samples were diluted in a 1: 10 ratio. Before carrying out photometric readings, the apparatus was calibrated using NaCl and KCl solutions of known concentrations.
The results obtained during the experimentation were subjected to a statistical treatment of a confidence level of 95%, using a variance analysis and an a posteriori test using Duncan´s simultaneous comparison method in order to determine significant differences between a) the average values of thermal survival and exposure time (days) for each acclimatization exchange rate used and b) the average values of concentrations of each ion and salinities measured in the tissues tested.
Results
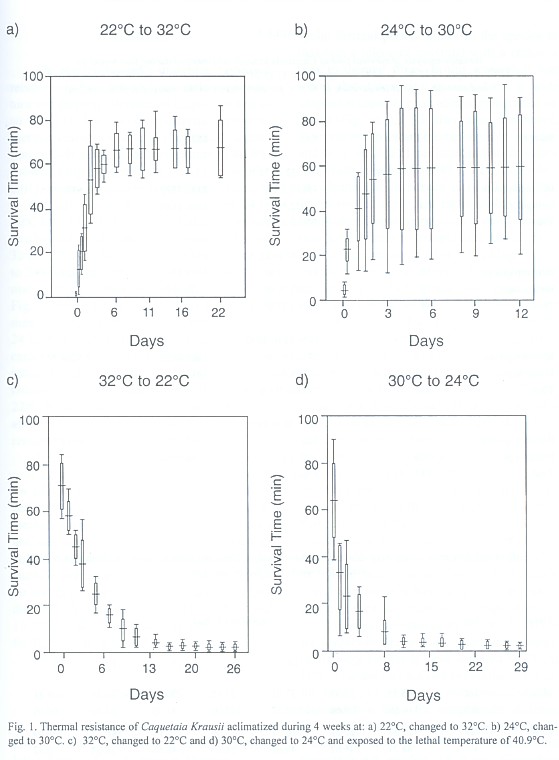
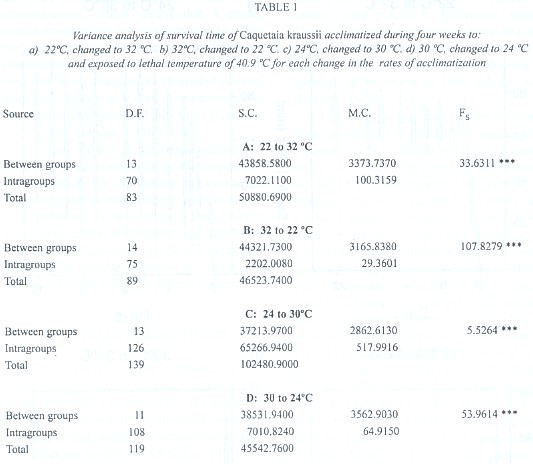
The results showed a straight and positive relationship between acclimatization temperature and thermal tolerance of Caquetaia kraussii (Fig. 1). The variance analysis applied to thermal resistance time during reacclimatization periods at the lethal temperature of 40.9ºC showed a highly significant effect (Anova, P < 0.01), on the increasing temperatures from 22 to 32ºC (df: 13/70; F: 33.63) and from 24 to 30ºC (df: 13/126, F: 5.53). Similar results were obtained for the decreasing conditions from 32 to 22ºC (df: 14/75; F: 107.83) and from 30 to 24ºC (df: 11/108; F: 53.96) for each thermal exchange rate (Table 1). An analysis of Fig. 1 showed that thermal resistance times measured before increasing temperatures from 24 to 30ºC and from 22 to 32ºC were significantly lower than those measured after temperature increased. On the other hand, for temperatures decreasing from 30 to 24ºC and 32 to 22ºC, thermal resistance times during 8-9 days after transfer were significantly higher than the remainder.
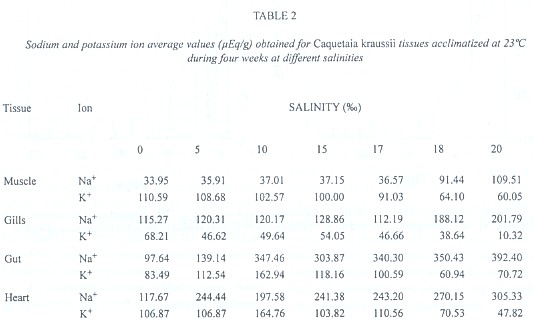
In Table 2, average values of ionic concentration of sodium and potassium are shown (in µEq/g) for Caquetaia kraussii, acclimatized at 23ºC and subjected to different environmental salinities during 4 weeks. The variance analysis show that a significant difference does not exist (Anova, P > 0.05) for the values related to sodium and potassium ions in muscular and gill tissues, but a significant difference did exist for gut (Fs = 52.247 for Na+ and 15.770 for K+) and heart tissues (Fs = 7.771 for Na+ and 6.71 for K+).
Discussion
The acclimatization involves a series of physiological changes within genetically regulated limits of each species (Segnini et al. 1993). The natural cycle of temperature produces a thermal resistance higher than constant acclimatization temperature in the laboratory (Ciurcina & Chung 1983). Because of that the cyclic thermal period allows to the species to maintain a tolerance maximal with a recovery time to thermal stress. In this research it was found that Caquetaia kraussiihave a wide adaptation capacity to an abrupt temperature increase (Fig. 1a, b): 3 h after being transferred, their thermal resistance time begin to increase and they reach full acclimatization at a new temperature regime from 48 to 72 h, depending on whether the increment is of 6ºC or 10ºC. These results imply that rapid acclimatization behavior of Caquetaia kraussii is due to adaptation mechanisms to temperature increases in their natural environment (La Aguá lagoon).
In the case of acclimatization to lowering temperatures, a fast decrease of thermal resistance is observed in those organisms exposed to a 6ºC decrease (Fig. 1c,d). When the acclimatization temperature difference is 10ºC, thermal resistance of this species diminishes slowly. This thermal decrease occurs abruptly at first (at 24 h), followed by two smaller decreases at a close interval (48 and 72 h), continuing with another remarkable descent (120 h) and later on, three small decreases (144, 216 and 288 h) until reaching full acclimatization at 336 h (14 days), where the variances are so small that they are not significant. On the other hand, in the 6ºC difference decrease, the thermal resistance time occurs in three stages: an abrupt loss in the first 24 h, then a marked descent from 2 to 4 days to conclude in the stability phase from that time onwards. Possibly, Caquetaia kraussii have a lesser capacity to diminish their acclimatization temperature.
The results obtained from 6ºC difference for Caquetaia kraussii acclimatization temperature were similar to those reported by Chung (1985) for another species belonging to the same family, Oreochromis mossambicus: they required two days for temperature increase, but acclimatization rate for temperature decrease was not similar. While Oreochromis mossambicus needed 14 days, Caquetaia kraussii only required 11 days. Segnini et al. (1993) reported one day to allow Mugil curema to reach their full acclimatization in a temperature increase from 24 to 30ºC and from 28 to 34ºC and 6 to 8 days in a temperature decrease at the same rates. The mechanism of different acclimatization types to temperature increase and decrease can be correlated with the fish´s metabolism, since temperature acts as a controlling factor of the state of molecular activation of the metabolic chain components (Wootton 1990), increasing in this way, the rate at which the chemical transformations take place. Therefore, decrease in water temperature diminishes the fish´s metabolism. On the other hand, a temperature increase speeds up its metabolism and, consequently, it is possible to assume that the acclimatization rate can be diminished at lower temperatures. The organisms that live in lagoons and shallow waters can be acclimatized to temperature changes during the day and they do not lose their acclimatization level during the night. These organisms have the advantage of tolerating higher temperatures, and they displace the thermal tolerance zone at high temperature levels (Bettinger & Fitzpatrick 1979, Chung & Strawn 1994).
On the other hand, it is necessary to note that Caquetaia kraussii acclimatized at 5, 10, 15 and 17 showed a 100% survival rate during the experimentation (40 days). When the salinity was changed at 18, the survival rate diminished to 97%, and at salinity 19, the survival rate was 95%. When the salinity was 20, the survival rate observed was 50% at 24 h and a 100% death toll at 36 h. The opercular movement stayed in a range between 74 and 76 bit/min, until salinity 15 and increased to 83-90 bit/min at higher salinities (19-20). The previous results show that the lethal incipient salinity for this species is 17. This is the limit at which they can be acclimatized, because beyond this concentration, the fish ingest little food. When the salinity was increased to 19, the fish stopped eating completely. When the salinity reached 20 the fish remained most of the time at the bottom of the aquarium and they were aggressive in defending their territory. If a fish lost a scale through the aggression of another fish, it ascended immediately to the surface of the water and it was almost immobile until it died. They presented a dark color, characteristic of a stress condition, as well as a slimy consistency. Their weight diminished 50%.
As we can observe, Na+ and K+ ions can regulate muscular and gill tissues, but they did not do so with gut and heart tissues. Caquetaia kraussii regulate the sodium and potassium ions separately but do not do both at the same time, mainly at 10 salinity. This seems to be the salinity limit in which this species begins to stimulate all its osmoregulatory system or stress strategy to compensate the salinity. Also, we can observe that Caquetaia kraussii abruptly modify their preferential temperature at this salinity. Segnini & Chung (1989) reported these changes (26.7 at 0, 25.6 at 5, 31.3 at 10 and 27.8ºC at 15) according to environmental salinity. These species modify their final preferendum (28.3 at 30.0ºC) when living in a hyperosmotic environment. These results confirm previous findings presented by Finstad et al. (1989) who pointed out that the ionic content in fish depends on temperature when the salinity is altered.
The processes of physiological adaptation in the gills to salinity changes are gradual and involve synthesis and destruction of molecular components of the epithelial transport system while monovalent ions (Na+ and K+) are excreted by the gills (Towle 1981, Langdon & Thorpe 1984).
At 5 and 10, 15 and 17 salinities, the physiological mechanisms of regulation to excrete the excess absorbed by the gills are stimulated, indicating that gills are highly permeable to sodium ions in both directions, and that saline balance is achieved by the adjustment of gain or loss of salts. All these mechanisms are very similar to the circulatory and hormonal system. They indicate that to maintain the sodium balance in teleost in a seawater environment, external potassium is necessary. It is thought that in seawater, the potassium ion is exchanged for the sodium ion which is eliminated through the blood into the seawater, and that this effect might be responsible for the maintenance of the sodium ion gradient concentration through the gills. The exchange suggests that activated adenosintrifosfatase by sodium and potassium (ATPase carrier) may have a function in the ionic exchange of the gills (Meador & Kelso 1990)
C. kraussii in salinities higher than 17 diminished the potassium levels in all the tissues studied, suffering a hypotacemia that brings the organism to death. These results confirm those reported by Robertson (1984), who studied the content of sodium and potassium in Anguilla anguilla muscle and found that sodium ion increased in the animals adapted to freshwater, even though the potassium ion diminished. For Caquetaia kraussii, potassium ion lowering was so significant that muscular paralysis occurred. The loss of potassium in the cell interior produces hyperpolarization of nervous and muscular tissues that prevent their transmission from the electric potentials to the nervous signals through these tissues.
From the results, it is possible to conclude that Caquetaia kraussii is an oligohaline fish that regulates the sodium and potassium ions at gills and muscle until the salinity reached (17), which is considered to be the incipient lethal level.
Acknowledgements
This work was supported by Research Council, Universidad de Oriente (CI-5-019-00642/94-97). We want to thank to Gordon Hart from University of Oriente for reviewing the manuscript.
Resumen
La sardina tropical de río, Caquetaia kraussii, capturada en la laguna La Aguá (Estado Sucre, Venezuela) fue aclimatada durante cuatro semanas a la temperatura de 22, 24, 30 y 32ºC y a 0, 5, 10, 15 y 17 de salinidad. Para evaluar los efectos de respuestas térmicas a los niveles de aclimatación, los peces fueron transferidos abruptamente desde las temperaturas bajas (22 y 24ºC) hasta las altas (32 y 30ºC) respectivamente. Se midió entonces la resistencia térmica a la temperatura letal de 40.9ºC durante 30 días. Se consideró que los peces habían alcanzado completamente su aclimatación cuando se estabilizaba al nuevo régimen de temperatura. Para el efecto salino, las concentraciones de sodio y potasio fueron determinadas en los tejidos: branquial, muscular, gástrico y cardíaco. Los resultados mostraron que la tolerancia térmica aumentaba rápidamente en 3 h cuando la diferencia de temperatura era de 6ºC (24 a 30ºC) y en 24 h cuando la diferencia era de 10ºC (22 a 32ºC). En los descensos de temperaturas, el nivel de aclimatación se alcanzó a los 11 días cuando el intercambio se hizo desde 30 a 24ºC y en 14 días con un descenso de 10ºC (32 a 22ºC). Los resultados indican que Caquetaia kraussii regula tanto sodio como potasio a nivel muscular y branquial a todos los niveles de salinidad estudiados; sin embargo a nivel gástrico y cardíaco se observaron regulaciones significativamente diferentes entre las salinidades examinadas.
References
Batttaglene, S.C. & R.B. Talbot. 1993. Effect of salinity and aeration on survival of an initial swim bladder inflation in larval Australian bass. Progr. Fish Cult. 55: 35-39. [ Links ]
Bennett, W.A. & F.W. Judd. 1992. Factors affecting the low temperature tolerance of Texas pinfish. Trans Am. Fish.Soc. 121: 659-666. [ Links ]
Bettinger, T.I. & L. Fitzpatrick. 1979. Physiological and ecological correlates of preferred temperature in fish. Zool. 49: 319-329. [ Links ]
Canahan, L.L., C.R. Feldmeth, J. Jones & D.L. Soltz. 1986. Energetic, salinity and temperature tolerance in the mohave tui chub, Gila bicolor mohavensis. Copeia 1: 45-52. [ Links ]
Chung, K.S. & K. Strawn. 1994. What factors influence the thermal tolerance of estuarine animal?. Interpretation of multiple regression analyses. Rev. Biol. Trop. 42: 365-370. [ Links ]
Chung, K.S. 1985. Adaptabilidad de Oerochromis mossambicus (Peters) 1852 a los cambios de temperatura. Acta Cient. Vzlana. 36: 180-190. [ Links ]
Ciurcina, P. & K.S. Chung. 1983. Efectos de la temperatura ambiental y la temperatura de aclimatación sobre la tolerancia térmica en ejemplares juveniles de la lisa Mugil curema. Bol. Inst. Oceánogr. Vzla. Univ. Oriente 22: 35-42. [ Links ]
Finstad, B., K.J. Nilseen & O.A. Gulseth. 1989. Seawater tolerance in freshwater resident artic charr (Salvelinus alpinus). Comp. Biochem. Physiol. 92A: 599-600. [ Links ]
Hurtado, S.N. 1975. Desarrollo embrionario del Pavón dorado Petenia kraussii Steindachner 1878 (Perciformes, Cichlidae). Estación Hidrobiológica de Guayana, Fundación La Salle de Ciencias Naturales. Contribución No. 1. pp. 309-319. [ Links ]
Langdon, J.S. & J.E. Thorpe. 1984. Response of the gill Na+ + K+ - ATPase activity, dehydrogenase activity and chloride cells to saltwater adaptation in Atlantic salmon, Salmo salar L. Parr and smolt. J. Fish Biol. 24: 323-331. [ Links ]
Meador, M.R. & W.E. Kelso. 1990. Physiological responses of largemouth bass, Micropterus salmoides, exposed to salinity.Can. J. Fish. Aquat. Sci. 47: 2358-2363. [ Links ]
Robertson, J.D. 1984. The blood plasma and parietal muscle of Oslo Fjord eels (Anguilla anguilla) and the river Lampetra fluviatilis L. Comp. Biochem. Physiol. 77A: 431-439. [ Links ]
Salman, N.A. & F.B. Eddy. 1988. Kidney function in response to salt feeding in rainbow trout (Salmo gairdneri R.) Comp. Biochem. Physiol. 89A: 535-539. [ Links ]
Segnini, M.I. & K.S. Chung. 1989. Influencia de la salinidad sobre el preferendum final de la mojarra de río (Petenia kraussii) Steindachner 1878 (Perciformes, Cichlidae). Bol. Inst. Oceanogr. Vzla. Univ. Oriente 28: 145-150. [ Links ]
Segnini, M.I., K.S. Chung & P. Ciurcina. 1993. Tasa de aclimatación al cambio de temperatura de Mugil curema (Pises: Mugilidae) de Venezuela. Rev. Biol. Trop. 41: 59-62. [ Links ]
Segnini de Bravo, M.I.& K.S. Chung, 1997. Influencia de los factores ambientales sobre el crecimiento instantáneo de peces tropicales evaluados por el seguimiento de la relación ARN/ADN. Bol. Inst. Oceánogr. Vzla. Univ. Oriente 36: 12-27. [ Links ]
Towle, D.W. 1981. Role of Na+ + K+ - ATPase in ionic regulation by marine and estuarine animals. Mar. Biol. Lett. 2: 107-122. [ Links ]
Wootton, R.J. 1990. Ecology of teleost fishes. Fish and Fisheries Series I. Chapman and Hall. London, England, 404 pp. [ Links ]
1 Instituto Oceanográfico de Venezuela, Dpto. Biología Marina, Universidad de Oriente, Núcleo de Sucre, Cumaná 6101, Venezuela. E-mail: fbravo@raudo.udo.udo.ve. Telefax: (5893) 512017, 512276.














