Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.49 n.1 San José Mar. 2001
Norma Ferriz-Domínguez1 and Guillermo Horta-Puga 2
Recibido 5-II-1999. Corregido 29-III-2000. Aceptado 31-VII-2000
Abstract
The short-term aggressive behavior of scleractinian corals from La Blanquilla Reef, Veracruz Reef System, Gulf of Mexico was determined. Unilateral aggression, bilateral aggression and indifference were observed through experimental interspecific encounters in situ, in aquarium conditions and through direct observation of natural encounters on the reef. Species were characterized as highly aggressive, moderately aggressive and not very aggressive establishing a hierarchy with two competitive rings representing similar aggressive abilities among some species. Most observations of aggression were of extracoelenteric digestion. Mussa angulosa was the most aggressive species and Oculina diffusa the least. Apparently there is a direct relationship among aggressiveness and relative coverage as three of the most abundant corals at dephts of 4-9 m Montastraea cavernosa, Colpophyllia natans and Montastraea annularis, are all highly aggressive, have massive growth and have a high relative coverage. Siderastrea siderea is the only dominant species that was not ranked as highly aggressive; its high coverage is due to other reasons.
Key words: Scleractinian, coral, aggression, behavior, Veracruz.
The available space in a coral reef is a limited resource (Bak 1975, Connell, 1976). As a result, the scleractinian corals have developed diverse mechanisms that allow them to gain and maintain the space that they occupy (Lang and Chornesky 1990). Corals may directly cause tissue damage in some neighboring organisms and possibly cause their death, mainly through aggression (Porter 1974, Lang and Chornesky 1990, Genin et al. 1994). Aggression is carried out by means of specialized structures called sweeper tentacles (Den Hartog 1977, Richardson et al. 1979, Wellington 1980, Chornesky 1983, Ates 1989), or through extracoelenteric digestion, using the mesenteric filaments of the gastrovascular cavity (Lang 1970, 1973, Logan 1984, Sheppard 1979, Cope 1981, Yamazato and Yeemin 1986). Field studies have been carried out in scleractinian corals, facing species against each other, to determine which species are more aggressive and therefore dominant (Lang 1970, 1973, Logan 1984, Sheppard 1979, Cope 1981, Yamazato and Yeemin 1986). These studies have allowed researchers to classify and to rank coral species as highly aggressive, moderately aggressive and not very aggressive. Complex and not always linear patterns of aggressive dominance have been established (Logan 1984). Active aggression by scleractinian corals contributes to the determination of reef coral community structure (Lang 1970, 1973; Sheppard 1980a, b, Bak et al. 1982, Lang and Chornesky 1990). Additionally some experiments in ahermatypic corals have demonstrated that a competitive dominant may influence the abundance and population structure of a subordinate by reducing its sexual reproductive output, increasing larval mortality and altering recruitment patterns (Chadwick 1991). Aggressive behavior has also been used as a taxonomic character to differentiate species of the same genus, as in Scolymia (Lang 1971), Acropora (Potts 1978) and Montastraea (Weil and Knowlton 1994).
Like other attributes (e.g. color, shape) the patterns of aggressive behavior can slightly vary for the same species in different localities. Lang (1971, 1973) proposed a linear hierarchy in the extra-coelenteric digestive dominance pattern of corals from Jamaica, while Logan (1984) found that there might not be a clear dominance among some species. These results suggest regional differences that should be assessed. The objective of the present study was to determine the pattern of short-term of aggressive dominance in scleractinian corals from La Blanquilla reef, Veracruz Reef System (VRS), based on observations of corals in aquarium conditions and in the field. The VRS is located at the western limit of the coral reef distribution in the Atlantic (Horta-Puga et al. 1997), which confers to it particular characteristics that make it different from the other reef areas previously studied in the Caribbean biogeographic area.
Materials and methods
The study was carried out between February 1987 and August 1988, on the Blanquilla reef (19° 13´ 35 N, 96° 05´ 54 W) in the VRS. The experimental design consisted of establishing interspecific encounters, in situ and in aquarium conditions, as much as possible, between each of the readily-available scleractinian species. Experimental pairs were formed by tying two colonies of different species together with plastic wire, in such a way that the living tissue of each coral were in direct contact. Care was take in order to not damage the soft tissues while tying corals each other. We replicated, when possible, each experimental pair at least once. After a variable lapse of time, we recorded the results of the interaction in each pair as: (1) unilateral aggression, when just one species showed signs of tissue necrosis, having a dominant (winner) and a subordinate species, (2) bilateral aggression, when both organisms presented significant damage in the contact area, implying that both species were aggressive, but neither dominated, and (3) indifference, determined when no specimen showed tissue damage, also indicating that neither was dominant. Our results for the Montastraea annularis complex (Weil and Knowlton 1994) were recorded without any distinction among the species involved, for obviously reasons.
Experimental determination of aggression under aquarium conditions: To carry out this part of the study, we manually gathered numerous healthy colonies, 10 cm apart from other corals, and with a diameter of >7 cm in the N, S and W slopes of the reef at depths of 4-10 m. We placed the colonies in polyethylene bags with sea water from the collection site, stirring the water occasionally and avoiding direct sunlight. The corals were taken to the lab in mainland within 30 min of collection, where they were placed in 40 l glass aquariums, each with a continuous flow of recirculated water, obtained from reef. The aquariums were located outdoors, indirectly exposed to the sun. The following day after collection, we formed experimental pairs, always keeping one colony of each species alone as control in order to detect if the aquarium environmental conditions were not the cause of tissue damage. In order to determine the behavior pattern of each species every 3 hours we checked the schedule of activity and the mechanism being used for aggression, if any, during a period of up to 192 hrs. According to Lang (1970) this is enough time to determine the extracoelenteric digestion responses of corals.
Experimental determination of the aggression on the reef: The field study was carried out at 4-9 m on the same N, E and W slopes of La Blanquilla reef. We collected numerous specimens and tied them underwater, forming experimental pairs, that were positioned as close as possible to the places at which they have been collected. We checked each of the 300 experimental pairs after 192 hrs to look for signs of aggressive behavior. As experimental controls, we placed healthy colonies in the same area, to verify that transplantion was not the reason for any observed responses.
Natural interactions observed in the reef: During the numerous dives carried out to collect the specimens for the study, we made direct observations of the natural interactions among corals in the reef. Each interaction was carefully observed and data such as: participating species and type of interaction were recorded.
Results
Aggressive behavior under aquarium conditions: We obtained results for 345 experimental pairs that implied interactions among 16 species, of which 280 gave conclusive results (Fig. 1). In the remaining pairs the corals died during the course of the experiment. Extracoelenteric digestion by the extrusion of the mesenteric filaments was the only observed mechanism used by the dominant colony to attack the subordinate species, causing tissue necrosis to the latter. In Agaricia agaricites, A. lamarcki, Leptoseris cucullata and Oculina diffusa, we did not see mesenteric filaments, even when they were dominant in different interspecific encounters. Chornesky (1983) recorded sweeper tentacles in Agaricia, but they were not observed here, possibly due to the fact that they only develop after several weeks of tissue contact. However, the damage inflicted by these species on the subordinate species was similar to that of the filament extruding species. Bak et al. (1982) reported that in Agaricia agaricites the filaments are very small and their observation is only possible under a microscope. Almost all the species extruded their mesenteric filaments during the night, generally 6-9 hrs after the beginning of the contact. Montastraea cavernosa and Colpophyllia natans were the only ones that extruded their filaments during the day. We observed three or four extruded filaments per polyp in most of the aggressive colonies. However, in some colonies of Montastraea cavernosa, there were as many as approximately 30 extruded filaments per polyp. The time for the manifestation of a clear aggressive dominance was variable for each species. Thus, while Montastraea cavernosa was dominant over Stephanocoenia intersepta in 24 hrs, Colpophyllia natans took up to 120 hrs to dominate this same species.
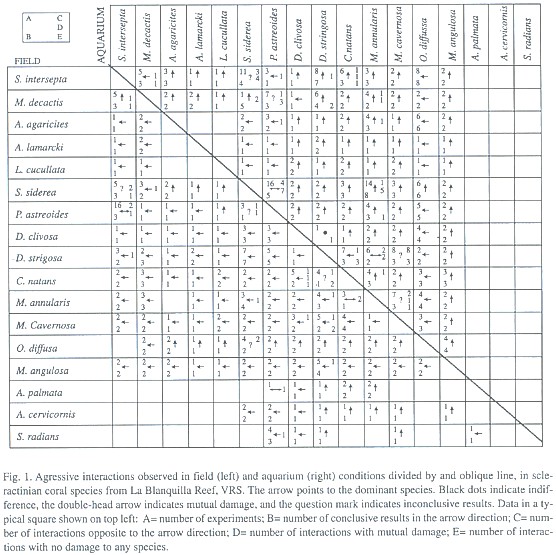
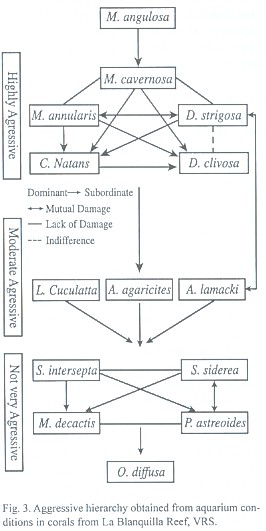
Based in the results of these experimental interspecific encounters, we determined a hierarchy of aggressive dominance (Fig. 3), in which the studied scleractinian species are classified as being highly aggressive, moderately aggressive or not very aggressive on the basis of their rank in the hierarchy and on the number of subordinate species. The hierarchy is not completely linear, since two groups with similar aggressive capacity were formed, implying the lack of a definitive dominance of any single species over the others. In these cases we observed bilateral damage, indifference or opposite results among the species of the corresponding in the replicates. Mussa angulosa was the most aggressive species, followed by the first group formed by Montastraea spp., Diploria spp. and Colpophyllia natans, which were also classified as highly aggressive. Agaricia agaricites, A. lamarcki and Leptoseris cucullata were classified as moderately aggressive species. Madracis decactis, Stephanocoenia intersepta, Porites astreoides, Siderastrea siderea and Oculina diffusa formed the second group and were not very aggressive species dominated by other species of corals. Species such as Acropora spp., Agaricia fragilis, Siderastrea radians, Porites porites, P. branneri, Favia conferta, Scolymia spp., Oculina valenciennesi and Astrangia solitaria, although present in the reef (Bravo and Camacho, 1989) were not included in this part of the study either because they did not survive the experimental conditions or were not found during the collection dives.
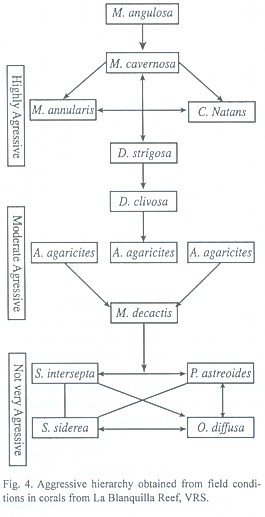
Aggressive behavior in field conditions: Conclusive results were obtained in 219 of 300 experimental pairs in the field (Fig. 1). Acropora cervicornis, A. palmata and Siderastrea radians were included in these studies. Some experimental pairs were separated by the action of surf, currents or fishes or other reef organisms. Contrary to our results for the aquarium conditions, it was not always possible to observe the aggression mechanism(s) used by every species. The resulting classification as highly aggressive, moderately aggressive and not very aggressive species is similar to that determined under aquarium conditions. There are slight differences on the arrangement of species within the competitive rings (Fig. 4). Acropora cervicornis, A. palmata and Siderastrea radians, although they are not show in the hierarchy, were considered not very aggressive since they were subordinate to the highly aggressive species, although due to the small number of experimental pairs for these species, it is not possible to draw conclusive results about their behavior.
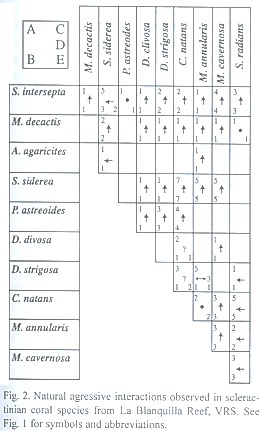
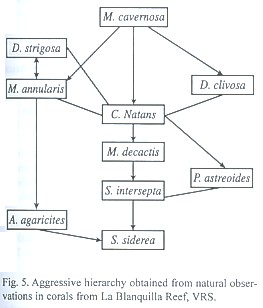
Records of natural interactions: We recorded 88 natural interactions among corals of 11 different species in La Blanquilla reef (Fig. 2). We only observed two interaction types, unilateral aggression or indifference. The hierarchy of natural aggresssive dominance using these observations (Fig. 5) closely resembles those obtained experimentally.
Discussion
Based on the results from field and aquarium observations, scleractinian corals of La Blanquilla reef can be classified in: (1) highly aggressive species: Mussa angulosa, Montastraea annularis complex, M. cavernosa, Diploria strigosa, D. clivosa and Colpophyllia natans, (2) moderately aggressive species: Agaricia agaricites, A. lamarcki and Leptoseris cucullata, and (3) not very aggressive species: Stephanocoenia intersepta, Siderastrea siderea, Madracis decactis, Porites astreoides, Oculina diffusa, Acropora palmata, A. cervicornis, Siderastrea radians, Porites branneri and Porites porites. This classification does not represent a strictly linear model of dominance (hierarchy), since some species in categories 1 and 3 showed similar capacities of interspecific aggression. Although we did not observe the development of sweeper tentacles or any other known aggression mechanism in any species, they could be involved in the interactions. Hence, a possible explanation for the lack of a definitive dominance among the coral species that formed the groups, is that more than one aggression mechanisms could be used. Thus, the ability of any species to compete could be variable (Lang, pers. comm.).
There were no important differences among the dominance patterns found in this study, and those previously noted in Jamaica (Lang 1970, 1973) and Bermuda (Logan 1984), and the more recent study in Curacao (Van Veghel et al. 1996), where the scleractinian fauna is similar. Lang (1973) ranked M. cavernosa lower in the hierarchy than Diploria spp. and M. annularis, and Logan (1984) ranked this sames species higher. This can be considered an evidence for our suggestion of the existence of a group of higher rank of similar aggressive capacities, in addition of that proposed for the weakly-aggressive species. Unfortunately, the absence of some species for each studied geographic area, could not permit a thorough comparison of the hierarchies. The small differences in the aggressive capacity registered for each species in each area, could be explained by: (1) the state of health of the used colonies, (2) physical, chemical, seasonal and/or geographical variations as was suggested by Logan (1984, 1986), and (3) the use of more than one mechanisms of aggression (Lang and Chornesky 1990).
The coral community structure in the reef slopes of La Blanquilla reef is well known (Bravo et al. 1989). The most abundant species on the reef slopes between 4-9 m depth in order of importance are: Montastraea cavernosa (28%), Colpophyllia natans (20%), Siderastrea siderea (20%) and Montastraea annularis (13%). Each of this species, except Siderastrea siderea, is characterized as highly aggressive. The aggressive character of these species could explain in part their high coverage in the reef, as was pointed out for Montastraea annularis complex by Van Veghel et al. (1996). All are also massive colonies that grow slower than ramose and leafy colonies, like Acropora and Agaricia respectivelly. Our results constitute an additional evidence that some corals use aggressive behavior to compete against the fast-growing species and each other, in order to gain and maintain the space in the reef (Lang 1970, 1973, Sheppard 1980a, b, Bak et al. 1982, Genin et al. 1994).
In the case of Siderastrea siderea, its high bottom coverage could be explained by its high reproductive potential (Szmant 1986) and its capacity to adapt to variable environmental conditions (Bak and Elgershuizen 1976, Loya, 1976). Mussa angulosa constitutes an exceptional case, although it is the most aggressive coral species, it is not abundant in La Blanquilla reef (relative coverage <<1%), thus, its aggressive capacity either play a secondary role in the determination of its coverage and/or distribution,and due that grows and reproduces slowly, without its high digestive dominance and ability to tolerate some shade, it might no longer exist at all.
The VRS is a marginal reef environment, that has lower faunal diversity (e.g. corals, fish, annelids, mollusks) than other Caribbean reef areas, indicating an impoverished ecosystem that has developed at the limit of tolerable environmental conditions possibly mediated by low winter temperatures and high sedimentation rates (Gutiérrez et al. 1993, Carricart-Ganivet and Horta-Puga 1993, Vargas-Hernández et al. 1993). These reefs have also been imperiled for centuries, from the arrival of the Spaniards at the beginning of the 16th century, to the present. The anthropogenic impacts on these reefs include chemical pollution, over-fishing, accidental oil spills, unrestricted tourism, ship groundings, discharges of sewage effluents, dredging, boat anchoring, and the extraction of coral for building purposes (Horta-Puga et al. 1997). In spite of this severe environmental framework for the VRS, the coral species exhibit the same aggressive capacities as in other reef areas of the Western Atlantic.
Acknowledgments
Many thanks to Judy Lang for her thoroughly review and her suggestions that notably improved the manuscript. We also thank Leonardo Alcantar, Juan Pablo Carricart, Ricardo Saenz, Joel Reyes, Jaime Camacho, Juan Bravo, Hugo Castro and Miguel Fuentes for their help in the experimental design and sampling. Thanks to Guillermina Sedas for her support during the field work.
Resumen
Se determinó el comportamiento agresivo a corto plazo de los corales escleractinios del arrecife La Blanquilla, Sistema Arrecifal Veracruzano, en el Golfo de México por medio de pares experimentales interespecíficos en condiciones de acuario y campo, así como de la observación directa de encuentros interespecíficos naturales. Las especies de coral fueron caracterizadas como altamente agresivas, moderadamente agresivas y no muy agresivas, estableciéndose una jerarquía en la que se incluyen dos anillos competitivos en los cuales las especies tienen una capacidad agresiva similar. La digestión extracelentérica fue el mecanismo de agresión más frecuentemente observado. La especie más agresiva fue Mussa angulosa y la menos agresiva Oculina diffusa. Aparentemente existe una relación directa entre la capacidad agresiva y la cobertura relativa de las tres especies más abundantes a profundidades entre 4-9 m Montastraea cavernosa, Colpophyllia natans y M. annularis, ya que todas fueron clasificadas como altamente agresivas, tienen un crecimiento masivo y una cobertura relativa alta. Siderastrea siderea es la única especie dominante que no fue clasificada como altamente agresiva, por lo que su cobertura alta es debida a otros factores.
Referencias
Ates, R. 1989. Aggressive behavior in corals. Freshwater Mar. Aquarium 12: 181-190. [ Links ]
Bak, R.P.M. 1975. Ecological aspects of the distribution of reef corals in the Netherland Antilles. Bijd. Dierk. 45: 181-190. [ Links ]
Bak, R.P.M. & J.H.B.W. Elgershuizen. 1976. Patterns of oil-sediment rejection in corals. Mar. Biol. 69: 215-222. [ Links ]
Bak, R.P.M., R.M. Termaat & R. Dekker. 1982. Complexity of coral interactions: Influence of time, location of interaction and epifauna. Mar. Biol. 69: 215-222. [ Links ]
Bravo-Ruiz, J., E. J. Camacho-Ruiz & J.P. Carricart-Ganivet. 1989. Composición de especies y zonación de corales escleractinios en el arrecife "La Blanquilla", Veracruz. Programa y resúmenes. X Congreso Nacional de Zoología, México, D.F. [ Links ]
Carricart-Ganivet, J.P. & G. Horta-Puga. 1993. Arrecifes de coral en México, p. 80-90. In S.I. Salazar-Vallejo, y N.E. González (eds.), Biodiversidad Marina y Costera de México. Com. Nal. Biodiv. y CIQRO, México, D.F. [ Links ]
Connell, J.H. 1976. Competitive interactions and the species diversity of corals, p. 51-58. In: G.O. Mackie, (ed.), Coelenterate ecology and behavior. Plenum Press, New York. [ Links ]
Cope, M. 1981. Interspecific coral interactions in Hong Kong. Proc. 4th Int. Coral Reef Symp., Manila, Philippines 2: 357-362. [ Links ]
Chadwick N E. 1991. Spatial distribution and the effects of competition on some temperate Scleractinia and Corallimorpharia. Mar. Ecol. Progr. Ser. 70: 39-48. [ Links ]
Chornesky E.A. 1983. Induced development of sweeper tentacles on the reef coral Agaricia agaricites: A response to direct competition. Biol. Bull. 165: 569-581. [ Links ]
Den Hartog, J.C. 1977. The marginal tentacles of Rhodactis sanctithomae (Corallimorpharia) and the sweeper tentacles of Montastrea cavernosa (Scleractinia); their cnidom and possible function. Proc. 3rd Int. Coral Reef Symp., Miami, Florida 1: 463-469. [ Links ]
Genin, A., L. Karp & A. Miroz. 1994. Effects of flow on competitive superiority in scleractinian corals. Limnol. Oceanogr. 39: 913-924. [ Links ]
Gutiérrez, D., C. García-Saez, M. Lara & C. Padilla. 1993. Comparación de Arrecifes coralinos Veracruz y Quintana Roo, p. 787-806. In S.I. Salazar-Vallejo y N.E. González (eds.), Biodiversidad Marina y Costera de México. Com. Nal. Biodiv. y CIQRO, México, D.F. [ Links ]
Horta-Puga, G., G. Barba-Santos & J.L. Tello-Musi. 1997. The Veracruz Reef System in the Gulf of Mexico: An Environmental Review. Symp. Ecosist. Acuat. Méx., México D.F., México. Programa: 015. [ Links ]
Lang, J.C. 1970. Inter-specific aggression within the scleractinian reef corals. Ph.D. Thesis, Yale Univ., New Haven, Connectticutt. 80 p. [ Links ]
Lang, J.C. 1971. Interspecific aggression by scleractinian corals. 1. The rediscovery of Scolymia cubensis (Milne-Edwards and Haime). Bull. Mar. Sci. 21: 952-959. [ Links ]
Lang, J.C. 1973. Interspecific aggression by scleractinian corals. 2. Why the race is not only to the swift. Bull. Mar. Sci. 23: 260-279. [ Links ]
Lang, J. C. & E.A. Chornesky. 1990. Competition between scleractinian reef corals- A review of mechanisms and effects, p. 209-252. In Dubinsky, Z. (Ed.). Coral Reefs. Ecosystems of the World 25. Elsevier, Amsterdam. [ Links ]
Logan, A. 1984. Interspecific aggression in hermatypic corals from Bermuda. Coral Reefs 3: 131-138. [ Links ]
Logan, A. 1986. Aggressive behavior in reef corals: A strategy for survival. Sea Frontiers/Sea Secrets 32: 347-351. [ Links ]
Loya, Y. 1976. Effects of water turbidity and sedimentation on the community structure of Puerto Rican corals. Bull. Mar. Sci. 26: 450-466. [ Links ]
Porter, J.W. 1974. Community structure of coral reefs on opposite side of the isthmus of Panama. Science 186: 543-545. [ Links ]
Potts, D.C. 1978. Differentiation in coral populations. Atoll Res. Bull. 220: 55-74. [ Links ]
Richardson, C.A., P. Dustan & J.C. Lang. 1979. Maintenance of living space by sweeper tentacles of Montastrea cavernosa, a Caribbean reef coral. Mar. Biol. 55: 181-186. [ Links ]
Sheppard, C.R.C. 1979. Interspecific aggression between reef corals with reference to their distribution. Mar. Ecol. Prog. Ser. 1: 237-247. [ Links ]
Sheppard, C.R.C. 1980a. Roles of interspecific and intraspecific competition on coral zonation. Progr. Underwater Sci. 6: 57-60. [ Links ]
Sheppard, C.R.C. 1980b. Coral cover, zonation and diversity on reef slopes of Chagos Atolls, and population structures of the major species. Mar. Ecol. Progr. Ser. 6: 57-60. [ Links ]
Szmant, A.M. 1986. Reproductive ecology of Caribbean reef corals. Coral Reefs 5: 43-54. [ Links ]
Tunnell, J.W. 1992. Natural versus human impacts to Southern Gulf of Mexico coral reef resources. Proc. 7th Int. Coral Reef Symp., Guam 1: 300-306. [ Links ]
Van Veghel, M.L.J., D.F.R. Cleary & R.P.M. Bak. 1996. Interspecific interactions and competitive ability of the polymorphic reef-building coral Montastrea annularis. Bull. Mar. Sci. 58: 792-803. [ Links ]
Vargas-Hernández, J.M., A. Hernández-Gutiérrez & L.F. Carrera-Parra. 1993. Sistema Arrecifal Veracruzano, p. 559-575. In: S.I. Salazar-Vallejo, y N.E. González (eds.), Biodiversidad Marina y Costera de México. Com. Nal. Biodiv. y CIQRO, México, D.F. [ Links ]
Weil, E. & N. Knowlton. 1994. A multi-character analysis of the caribbean coral Montastraea annularis (Ellis and Solander, 1786) and its two sibling species, M-faveolata (Ellis and Solander, 1786) and M-franksi (Gregory, 1895). Bull. Mar. Sci. 55: 151-175. [ Links ]
Wellington, G.M. 1980. Reversal digestive interactions between pacific reef corals: Mediation by sweeper tentacles. Oecologia 65: 516-525. [ Links ]
Yamazato, K. & T. Yeemin. 1986. Preliminary study on the inter- and intraspecific interactions among corals of Khang Khao Island, the Sichang Islands, Gulf of Thailand. Galaxea, 5: 163-174 [ Links ]
1 Pronatura Península de Yucatán, A.C. Calle No. 1-D 254-A. Col. Campestre. Mérida, Yucatán 97120, México; ppy@pibil.finred.com.mx
2 INVEMAR, UBIPRO, ENEP Iztacala, Universidad Nacional Autónoma de México. Av. de los Barrios s/n, Los Reyes Iztacala, Tlalnepantla, México 54090. Fax: +(52) 56231225; horta@servidor.unam.mx














