Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.48 n.4 San José Dec. 2000
Maria Stela Maioli Castilho and Marlene Sofia Arcifa1
Received: 15-VI-00 Corrected: 7-IV-00 Accepted: 24-V-00
Abstract
Secondary production, density and biomass fluctuations, and some reproductive aspects of the rotifer Brachionus plicatilis were studied weekly for two years in a Brazilian coastal lagoon. Food (and secondarily temperature) seemed to be the main factor involved in the summer increments. In the first summer, a mass mortality of fish caused changes in the phytoplankton, which switched from the dominance of Cyanophyceae to Chlorophyceae and other groups. For two months, production, density and biomass were relatively high until the recovery of the Cyanophyceae dominance. In the second summer, peaks of shorter duration and higher values of production, in comparison to the first one, were found. The contribution of resting eggs hatching, in the first summer, and the increment in parthenogenetic reproduction, in the second summer, would account for the population increases observed. Males and mictic females occurred when densities were high.
Key words
coastal lagoon, zooplankton, Brachionus plicatilis, secondary production, mictic phase, South America.
Introduction
The rotifer Brachionusplicatilis (O.F. Müller,1786) is a cosmopolitan species (Walker 1981) commonly used to rear fish larvae, which explains the widespread interest on this organism. However, most publications refer to laboratory experiments and only a few to natural environments (Walker 1973, 1981). Some aspects studied are: nutrition (Vadstein et al. 1993, Yúfera and Navarro 1995), physiology (Epp and Winston 1978, Miracle and Serra 1989), reproduction (Lubzens et al. 1993, Carmona et al. 1995) and genetic variability aspects among populations (Serra and Miracle 1987).
Because B. plicatilis tolerates large variations in environmental conditions as an osmoconformist (Epp and Winston 1977), it is dominant in some stressed environments, such as Barra Lagoon (Arcifa et al. 1994).
Zooplankton composition and fluctuations in Barra Lagoon were studied from December 1990 through August 1993, and were partially published (Arcifa et al. 1994). The present paper focuses on secondary production, density and biomass fluctuations, egg production, and appearance of males and mictic females of Brachionus plicatilis, from December 1990 to May 1992.
Materials and methods
Barra Lagoon (22º57 S, 42º47 W) is part of a coastal lagoon system, the Maricá System, located in Rio de Janeiro State, Brazil. Due to the influence of the sea and freshwater sources, salinity varies from 3 to 12 in this lagoon.
Data presented here refer to the period from December 1990 to May 1992 and are part of a zooplankton study in Barra Lagoon. The study was divided into Period I (December/90 - June/91) and Period II (October/91 - May/92).
Samples were taken weekly from one station with an electric bilge pump, delivering 30 l/min, and the water (210 l) was filtered through a 60 µm mesh net. Samples were preserved in 4 % formaldehyde.
Zooplankton organisms were counted in 1 ml subsamples in a counting chamber until attaining at least 60 individuals, as recommended by McCauley (1984). Two forms (1 and 2) were identified and counted separately.
Brood size was evaluated by counting the eggs of 30 randomly chosen egg-bearing females per sample.
Wet weight biomass was estimated through biovolume, based on the geometric form of the organism, considering density as 1 (McCauley 1984) and dry weight as 10 % of the wet weight (Doohan 1973). A total of twenty individuals was measured.
Secondary production was calculated with the equation proposed by Rigler and Downing (1984).
Results
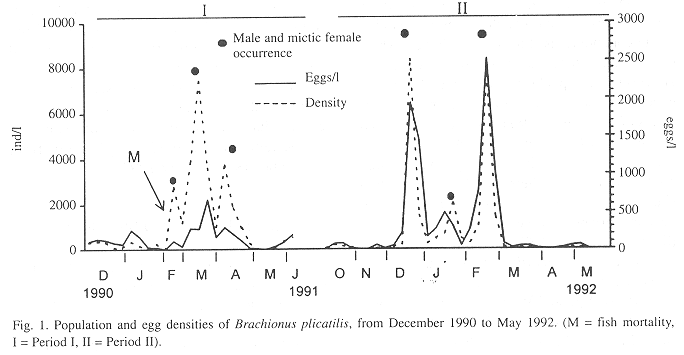
Densities and number of eggs of B. plicatilis were low during December 1990 (Period I) (Fig. 1). In January 1991, the number of eggs increased, but high densities (2774 ind/l) occurred only after a fish mortality in February 9 to 13. In March, after a decrease to ca. 1000 ind/l, population density increased again, reaching 7440 ind/l. In April, a drop to 1000 ind/l was followed by a new population growth, resulting in densities of 3700 ind/l. At the end of this month, density decreased again to 280 ind/l. In this phase (February - April/91) a new larger form of B. plicatilis, Form 2, occurred (Table 1).
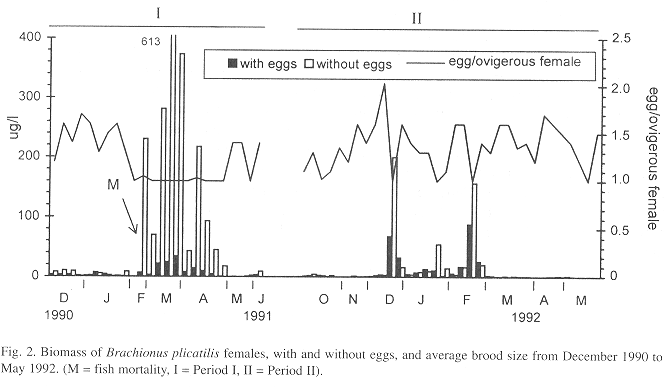
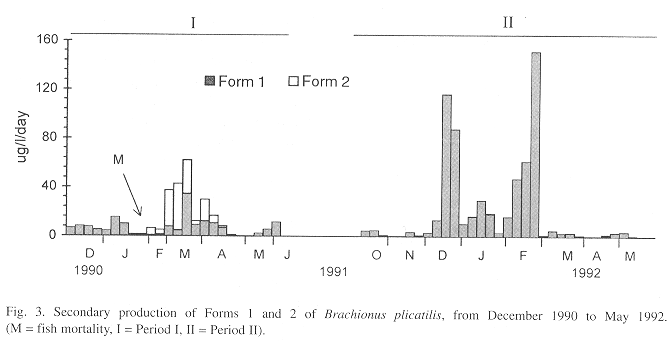
From February to April 1991, in comparison with the high densities (Fig. 1) and biomass (Fig. 2), secondary production of the two forms was relatively low (maximum of ca. 60 µg/l/day) (Fig. 3). Egg production was also low as indicated by the biomass values of egg-bearing females and brood size, which hardly exceeded 1 (Fig. 2). In May, densities (Fig. 1), biomass (Fig. 2), and secondary production (Fig. 3) were low.
Period II was also characterized by high B. plicatilis densities and biomass, in summer (December/91 - February/92) (Figs. 1 and 2). The high density of December (8375 ind/l) was followed by a sharp decrease to 208 ind/l. In January, population increased to 2036 ind/l and dropped again to ca. 200 ind/l. In February, a new peak of 7659 ind/l was recorded but, by the end of the month, density dropped to 108 ind/l. In Period II densities were higher than those of Period I, but biomass was lower, due to the absence of Form 2.
The number of eggs/l was higher in the second period than in the first one (Fig. 1), resulting in a higher secondary production (Fig. 3). The contribution of egg-bearing females and brood size were also higher in Period II than in Period I (Fig. 2).
Statistically significant inverse Spearman rank correlation was found between number of eggs and Cyanophyceae densities during Period I (r = -0.52; n = 24; P = 0.01) and also between salinity and B. plicatilis density during Period II (r = -0.36; n = 32; P = 0.04). Other correlations between number of eggs and some factors, such as temperature, salinity, Chlorophyceae, Cyanophyceae, and between B. plicatilis density and Cyanophyceae, Chlorophyceae and temperature, were not statistically significant.
Males and mictic females were found in samples during peaks of population density, in both periods (Fig. 1).
Discussion
Comparing Periods I and II, common features were the increase in density, biomass, and production of Brachionus plicatilis during summer, the occurrence of males and mictic females, and a brood size of one egg/female during the population peaks. Other features, such as number of eggs/l, ovigerous female: non-ovigerous female ratio, production and biomass values, and the duration of the summer peaks differed in both periods.
The average temperatures were relatively high during the whole study period (Table 2), reaching peaks of 30°C during summer. This fact would favour the rotifer population growth in this season, as it is known that higher temperatures promote an increase in population growth, egg production rates, and fertility of B. plicatilis (King and Miracle 1980, Snell 1986, Miracle and Serra 1989, Serra et al. 1994,).
It is possible, however, that food might be the main factor promoting population and production increases in the lagoon. The mass mortality of fish, in February 1991 (Period I), was responsible for changes in abiotic and biotic factors (Carmouze et al. 1994a, b; Domingos et al. 1994). In the phytoplankton, the dominant small Cyanophyceae (2.4 µm) - Synechocystis aquatilis f. salina and Synechococcuselongatus - were initially replaced by Chlorophyceae, such as Chlorella vulgaris var. autotrophica and C. minutissima (5.5 µm), and later also by Prasinophyceae, Bacillariophyceae, Cryptophyceae, and Dinophyceae. Chlorella, and part of the other algal groups possibly favoured the maintainance, for two months, of high densities, biomass, and production of Brachionus plicatilis, whose decrease coincided with the recovery of the same former Cyanophyceae, in April (Domingos et al. 1994).
Chlorella has been more successfully used for rearing this rotifer than Synechococcus (e.g.Snell 1986, James and Abu-Reseq 1988, Lubzens et al. 1993). Species of the latter, as well as Cyanophyceae in general, are considered a low-quality food, deficient in essential highly unsaturated fatty acids (DeMott and Müller-Navarra 1997, Gulati and DeMott 1997). The small size of Synechococcus and Synechocystis could also be inadequate, as preferences for particles in the range of 3 to 10 µm were reported for B. plicatilis (Spittler and Cruz S. 1988, Andres 1996).
Increases in density, biomass, and production in the summer of Period II are more difficult to explain based on phytoplankton composition, as it was dominated by the same small Cyanophyceae. But the duration of the peaks was shorter, suggesting suitable but ephemeral conditions, not related to phytoplankton. Possibly, other food resources were exploited.
The decrease of the average brood size to one egg/female, in both periods, coincided with population increases. In Period I, high total zooplankton densities, resulting in higher grazing pressure, seemed to be responsible for controlling phytoplankton growth, in spite of abiotic suitable conditions, including nutrients (Arcifa et al. 1994). Even so, relatively high algal densities and biomass were reported - 2.0-3.8 x 105 ind.ml-1 and 2-6 mgC.l-1, respectively (Domingos et al. 1994), making unlikely a limiting resource for the rotifer growth or a negative influence on its life table parameters. It is more plausible that a density-dependent control mechanism of the B. plicatilis population occurred, in this period, as well as in Period II.
It is probable that the appearance of males and mictic females, at the time of high rotifer densities, in both periods, has been caused by overcrowding, according to observations made in experiments (Pourriot and Snell 1983, Snell and Boyer 1988, Lubzens et al. 1993), and in the field (Walker 1973).
Taking into account both the relatively low number of eggs/l and of egg-bearing females, from February to April 1991, in Period I, it is reasonable to suppose that the contribution of resting eggs hatching of both rotifer forms was fundamental for the high population densities attained at that time. Resting eggs, mixed with mollusc fecal pellets, were found two months before the start of the rotifer increase. The hatching of resting eggs is related to organic matter photolysis by UVB rays, producing OH and resulting in biochemical influences on the eggs (Mopper and Zhou 1990, Hagiwara et al. 1995). Before fish mortality, dissolved organic carbon increased in the lagoon (Carmouze et al. 1994b), probably favouring the hatching of eggs. According to results of Minkoff et al. (1983) and Snell and Persoone (1989), light, temperature, and food conditions were also favourable to the hatching. In Period II, on the contrary, both the number of eggs/l and the ovigerous female: non-ovigerous female ratio were high. Therefore, it seems that the significant contribution of resting eggs hatching, in Period I, and the parthenogenetic reproduction, in Period II, would account for the population increases in the lagoon.
Form 2 was responsible for higher biomass values in Period I, in comparison to Period II. A higher production, in the second period, resulted from a larger number of eggs produced by more reproductive females.
Although there was a significant negative correlation between B. plicatilis density and salinity, in Period II, it is hard to explain its influence, considering that this rotifer species is euryhaline, surviving in salinities ranging from 1 to 97 (Walker 1981).
Acknowledgements
We thank J.-P. Carmouze, V.L.M. Huszar, P. Domingos, and M. Bernardes for supplying physical, chemical and biological data. We thank them and the students of the Aquatic Biochemistry Laboratory - Dept. of Geochemistry-UFF, responsible for samplings in Barra Lagoon. We also thank Walter Koste for the rotifer identification, FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for a research grant to M.S. Arcifa (nº 91/3873-4), and FAPESP and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for grants to the senior author (M.S.M.Castilho). Finally, we thank the comments of three anonymous referees.
References
Arcifa, M.S., M.S.M. Castilho & J.-P. Carmouze. 1994. Composition et évolution du zooplancton dans une lagune tropicale (Brésil) au cours d´une période marquée par une mortalité de poissons. Rev. Hydrobiol. trop. 27: 251-263. [ Links ]
Andres, N.N. 1996. Cultivo del rotífero Brachionus plicatilis O.F. Müller alimentado con microalgas liofilizadas. Dinámica de la población y calidad nutritiva como alimento larvario de peces marinos. Ph.D. Thesis, Universidad de Cadiz, Cadiz, Spain. 257 p. [ Links ]
Bernardes, M.C. 1995. As atividades biológicas relacionadas com o C, N e P das diferantes frações da matéria orgânica durante um ciclo anual na Lagoa da Barra, Maricá, RJ, Brasil. Ms. Thesis, Universidade Federal Fluminense, Niterói. Brazil. 138 p. [ Links ]
Carmona, M.J., A. Gomez & M. Serra. 1995. Mictic patterns of the rotifer Brachionus plicatilis Müller in small ponds. Hydrobiologia 313/314: 365-371. [ Links ]
Carmouze, J.-P., B. Farias & P. Domingos. 1994a. Évolution du métabolisme dune lagune tropicale (Brésil) au cours dune période marquée par une mortalité de poissons. Rev. Hydrobiol. trop. 27: 199-215. [ Links ]
Carmouze, J.-P., C. dÉlia Sampaio & P. Domingos. 1994b. Évolution des stocks de matière organique et de nutriments dans une lagune tropicale (Brésil) au cours dune période marquée par une mortalité de poissons. Rev. Hydrobiol. trop. 27: 217-234. [ Links ]
DeMott, W.R. & D.C. Müller-Navarra. 1997. The importance of highly unsaturated fatty acids in zooplankton nutrition: evidence from experiments with Daphnia, a cyanobacterium and lipid emulsions. Freshwat. Biol. 38: 649-664. [ Links ]
Domingos, P., V.L.M. Huszar & J.-P. Carmouze 1994. Composition et biomasse du phytoplancton dune lagune tropicale (Brésil) au cours dune période marquée par une mortalité de poissons. Rev. Hydrobiol. trop. 27: 235-250. [ Links ]
Doohan, M. 1973. An energy budget for adult Brachionus plicatilis Muller (Rotatoria). Oecologia (Berl.) 13 : 351-362. [ Links ]
Epp, R. & P.W. Winston. 1977. Osmotic regulation in the brackish water rotifer Brachionus plicatilis (Muller). J. Exp. Biol. 68: 151-156. [ Links ]
Epp, R. & P.W. Winston. 1978. The effect of salinity and pH on the activity and oxygen consumption of Brachionus plicatilis (Rotatoria). Comp. Biochem. Physiol. 59A: 9-12. [ Links ]
Gulati, R.D. & W.R. DeMott. 1997. The role of food quality for zooplankton: remarks on the state-of-the-art, perspectives and priorities. Freshwat. Biol. 38: 753-768. [ Links ]
Hagiwara, A., N. Hoshi, F. Kawahara, K. Tominaga & K. Hirayama. 1995. Resting eggs of the marine rotifer Brachionus plicatilis Müller: development, and effect of irradiation on hatching. Hydrobiologia 313/314: 223-229. [ Links ]
James, C.M. & T.S. Abu-Rezeq. 1988. Effects of different cell densities of Chlorella capsulata and a marine Chlorella sp for feeding the rotifer Brachionus plicatilis. Aquaculture 69: 43-56. [ Links ]
King, C.E. & M.R.Miracle. 1980. A perspective on aging on rotifers. Hydrobiologia 73: 13-19. [ Links ]
Lubzens, E., Y. Wax, G. Minko & F. Adler. 1993. A model evaluating the contribution of environmental factors to the production of resting eggs in the rotifer Brachionus plicatilis. Hydrobiologia 255/256: 127-138. [ Links ]
McCauley, E. 1984. The estimation of the abundance and biomass of zooplankton in samples, p.228-265. In J.A. Downing & F.H. Rigler (eds.). A manual on methods for the assessment of secondary productivity in fresh water. Blackwell Scientific, London. [ Links ]
Minkoff, G., E. Lubzens & D. Kahan. 1983. Environmental factors affecting hatching of rotifers (Brachionus plicatilis) resting eggs. Hydrobiologia 104: 61-69. [ Links ]
Miracle, M.R. & M. Serra. 1989. Salinity and temperature influence in rotifer life history characteristics. Hydrobiologia 186/187: 81-102. [ Links ]
Mopper, K & X. Zhou. 1990. Hydroxyl radical photoproduction in the sea and its potential impact on marine processes. Science 250: 661-664. [ Links ]
Pourriot, R. & T.W. Snell. 1983. Resting eggs in rotifers. Hydrobiologia 104: 213-224. [ Links ]
Rigler, F.H. & J.A. Downing. 1984. The calculation of the secondary productivity, p.19-58. In: J.A. Downing & F.H. Rigler (eds.). A manual on methods for the assessment of secondary productivity in fresh water. Blackwell Scientific, London. [ Links ]
Serra, M., M.J. Carmona & M.R. Miracle. 1994. Survival analysis of three clones of Brachionus plicatilis (Rotifera). Hydrobiologia 277: 97-105. [ Links ]
Serra, M. & M.R. Miracle. 1987. Biometric variation in three strains of Brachionus plicatilis as a direct response to abiotic variables. Hydrobiologia 147: 83-89. [ Links ]
Snell, T.W. 1986. Effect of temperature, salinity and food level on sexual and asexual reproduction in Brachionus plicatilis (Rotifera). Mar. Biol. 92: 157-162. [ Links ]
Snell, T.W. & E.M. Boyer. 1988. Thresholds for mictic female production in the rotifer Brachionus plicatilis (Muller). J. Exp. Mar. Biol. Ecol. 124: 73-85. [ Links ]
Snell, T.W. & G. Persoone. 1989. Acute toxicity bioassays using Rotifers. I. A test for brackish and marine environments with Brachionus plicatilis. Aquat. Toxicol. 14: 65-80. [ Links ]
Spittler, P. & Cruz. 1988. La selección del tamaño de las partículas alimenticias por Brachionus plicatilis (Rotifera). Rev. Investig. Mar. 9: 91-96. [ Links ]
Vadstein, O., G. Oie & Y. Olsen. 1993. Particle size dependent feeding by the rotifer Brachionus plicatilis. Hydrobiologia 255/256: 261-267. [ Links ]
Walker, K.F. 1973. Studies on a saline lake ecosystem. Aust. J. Mar. Freshwat. Res. 24: 21-71. [ Links ]
Walker, K.F. 1981. A synopsis of ecological information on the saline lake rotifer Brachionus plicatilis Müller 1786. Hydrobiologia 81: 159-167. [ Links ]
Yúfera, M. & N. Navarro. 1995. Population growth dynamics of the rotifer Brachionusplicatilis cultured in non-limiting food condition. Hydrobiologia 313/314: 399-405. [ Links ]
Table 1
Occurrence and average length and biomass of the two Brachionus
plicatilis forms from December 1990 to May 1992.
| | | Length ± s (µm) N=20 | Biomass ± s (µg) (dry weight) N=20 | |
| Rotifera B. plicatilis | ||||
| Form 1 | | | 116.72 ± 10.32 | 0.035 ± 0.009 |
| Form 2 | | | 171.99 ± 12.05 | 0.113 ± 0.040 |
Table 2
Month average values of salinity, temperature,
and phytoplankton density in Periods I and II.
| | | | ||
| | | | ||
| P I | ||||
| Dec/90 | | | | |
| Jan/91 | | | | |
| Feb | | | | |
| Mar | | | | |
| Apr | | | | |
| May | | | | |
| Oct | | | | |
| Nov | | | | |
| Dec | | | | |
| Jan/92 | | | | |
| Feb | | | | |
| Mar | | | | |
| Apr | | | | |
| May | | | | |
1 Departamento de Biologia, F.F.C.L.R.P., Universidade de São Paulo, Av. Bandeirantes 3900, 14040-901 Ribeirão Preto - SP, Brazil. fax: 55-16-602-3832 e-mail: mstela@usp.br.