Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.47 n.4 San José Dec. 1999
Helena C. Morais1 Ivone R. Diniz2 and Delano M. S. Silva1
Received 23-III-1998. Corrected 2-VII-1999. Accepted 8-VII-1999.
Abstract
We describe the seasonal abundance of caterpillars in the central Brazilian cerrado (savanna woodland) from 1991 to 1995 and discuss possible explanations for abundance variation. The climate is highly seasonal with a marked wet season between October and March. On 15% of a total of 10 800 censuses of host plants (nine species, three genera and three families) we found caterpillars feeding externally. We successfully reared 247 lepidopteran species (32 families), 61 % of which occurred at low density (fewer than four times). The frequency of plants with caterpillars increased sharply at the beginning of the dry season, in mid-April, and remained high until mid-July and then declined sharply, reaching its lowest point in the early-wet season (October). Some caterpillar species are present on food plants throughout the year, whereas others are highly seasonal, occurring only in the wet season. The duration of the pupal stage and its variance increased at the end of the wet season. Possible explanations for the low caterpillar density at the beginning of the rainy season include accumulated water stress in the late-dry season, leaf defenses, delay of adult emergence, and predator and parasitoid activities. The higher caterpillar abundance during the first half of the dry season may correspond to the availability of temporary enemy-free space.
Key words
Caterpillar abundance, dry season, Lepidoptera, herbivores, host plants, cerrado, Brazil.
Much is known concerning the influence of the dry season on the abundance and life cycles of tropical insects in seasonally dry tropical forests (see Denlinger 1986, Wolda 1988, Janzen 1993 for references). Many tropical insect populations vary seasonally and variation has often been correlated with rainfall, the highest abundance occurring in the wet season (e. g. Janzen 1973, Wolda 1988). In seasonal forest a number of lepidopteran species may diapause as final instar larvae inside pupal cocoons and then pupate at the following wet season (Aiello 1992). Others may pass the dry season as pupae and emerge as adults during the initial rains of a future wet season (Janzen 1987). Certain butterflies spend the dry season as adults in reproductive diapause (DeVries 1987), while others breed continuously, expanding and contracting their geographical distributions in response to larval food plant availability (Jones and Rienks 1987).
Knowledge concerning seasonal abundance fluctuations of tropical insect populations relies on several studies on adult insects and generally is restricted to only a few species. However, there is a general lack of studies regarding seasonal activities of the immature stadia of insects. Caterpillar seasonality is particularly evident in the tropical dry forest of Costa Rica, where caterpillar fauna fluctuates enormously in biomass and proportional species composition within a year (Janzen 1993).
In contrast to the various studies for seasonally dry forest of the neotropics, there are no studies of caterpillar seasonality in the neotropical savanna. The central Brazilian cerrado (edaphic woodland savanna) is highly seasonal, with marked wet and dry seasons, and most plant species are only briefly deciduous, despite the accentuated seasonality in rainfall. The cerrado region exhibits a very rich lepidopteran fauna, with around 1,000 butterfly species (Brown and Mielke 1967). It has been estimated that the moth fauna ranges from 5,000 to 8,000 species (V. O. Becker pers. com.). Plant-herbivore interactions may be qualitatively different from those of tropical deciduous forests because leaves remain on plants throughout much of the dry season.
The present work describes the seasonal abundance of caterpillars, the temporal occurrence of caterpillar species, and the duration of the pupal stage for species collected in the field at different times of the year. This study represents a first description of the seasonality of caterpillar abundance for the Brazilian cerrado, and includes some of the first data available on seasonal variation in species composition for any animal group in a cerrado area.
Materials and Methods
The field work was conducted in four one-ha blocks of cerrado sensu stricto (Goodland 1971) within three preserved areas near Brasília, DF, Brazil (15º 45 S, 47º 50 W): University of Brasília Experimental Farm (Fazenda Água Limpa - FAL), Botanic Garden of Brasília (Jardim Botânico de Brasília - JBB), and National Park of Brasília (Parque Nacional de Brasília - PNB). Detailed information on the vegetation of these sites can be found in Ratter (1980), Felfili and Silva (1993) and Felfili et al. (1993).
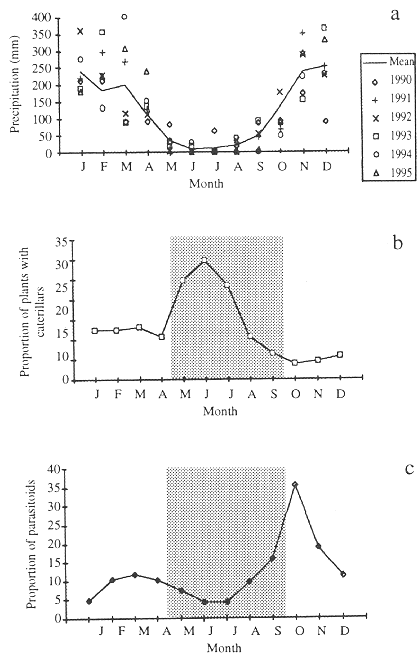
Brasília is at an altitude of approximately 1,000 m and rainfall is concentrated in the six months between October and March, with an average monthly precipitation of 207 mm. The dry months, from May through September, have a mean monthly precipitation of 24 mm. The precipitation varies among years (Fig. 1a).
Although the majority of the woody cerrado plant species are deciduous, flushing their new leaves between September and October at the beginning of the rainy season (Mantovani and Martins 1988; Morais et al. 1995), this occurs in a gradual asynchronous process among individuals of the same species and between species, and many species retain their leaves throughout the dry season (evergreen and semideciduous). Some species lose old leaves and flush new ones synchronously, remaining leafless for about one month during the dry season. This is the case for three of the host plant species examined in the present study (Erythroxylum deciduum, E. tortuosum and Qualea grandiflora).
From April 1991 to April 1993, all caterpillars encountered on any dicotyledonous host plants in 1 ha block at FAL were collected. Starting in May 1993 to May 1995 the number of examined plant species was reduced to nine. Fifteen individuals of each of the nine host plant species in three genera were examined for caterpillars once a week in FAL, JBB and PNB: Byrsonima coccolobifolia (Spr.) Kunth, B. crassa Nied., B. verbascifolia L. Rich. (Malpighiaceae), Erythroxylum deciduum St. Hill, E. suberosum St. Hill, E. tortuosum Mart. (Erythroxylaceae), Qualea grandiflora Mart., Q. multiflora Mart., and Q. parviflora Mart. (Vochysiaceae). For all species, individual plants examined varied from 0.50 to 2.50 m in height. The plants were censused once a week during morning hours from 8:00 to 12:00 am.
Externally-feeding caterpillars were collected and individually reared in plastic jars on leaves of the plant on which they were found. The duration of the pupal stage, and parasitoid emergence were accompanied under uncontrolled conditions (temperature, humidity, light) in the laboratory. The durations of the pupal period were pooled by month for each species. The monthly means averaged over all species were used. This procedure reduces the influence of those species that may occur abundantly during specific months.
Caterpillars were classified as morphospecies, but because the identification of morphospecies among collectors was not always consistent, for the analyses of temporal species composition we considered only the species that emerged as adults. This results in an underestimate of species occurrence. The adults were identified by Vitor O. Becker (EMBRAPA - Brasília) and Keith S. Brown Jr. (UNICAMP). Voucher specimens were deposited in the Departamento de Zoologia Collection at the Universidade de Brasília.
Results
Of the approximately 4,000 caterpillars collected in 80 species, 61 genera and 41 families of plants, we successfully reared about 1,500 lepidopteran adults representing 247 species from 32 families. Lists of species of caterpillars and their host plants are presented by Diniz and Morais (1995). One-hundred-fifty-one (61%) of the 247 lepidopteran species occurred at low density (less than four times in four years).
Between May 1993 and 1995, we conducted 10,800 censuses of nine host plants species, 15% of which had externally-feeding caterpillars. The 137 lepidopteran species (24 families) reared from the three genera of cerrado host plants during the quantitative survey are listed in Diniz and Morais (1997).
Caterpillar abundance (expressed as the proportion of plants with caterpillars) increased sharply in mid-April and remained high until mid-July, during the first half of the dry season, and then declined sharply until the lowest abundance in early-wet season (October) (Fig. 1b). The decrease in caterpillar abundance coincided with the second half of the dry season in the cerrado region (Fig. 1a), during which the host plants are leafless or have senescent leaves (August-September).
The most frequent species tended to occur throughout the year (see Table 1 for examples). These multivoltine lepidopteran species belong to various taxonomic groups and may be polyphagous or restricted to one plant genus in the study areas (Diniz and Morais 1995, 1997).
Fig. 1. A) Mean monthly precipitation for Brasília, DF, Brazil (1980-1995). Data from the IBGE meteorological station. Mean annual precipitation = 1,469 mm; Mean annual temperature = 21 ºC. B) Percentage of plants with caterpillars. C) Percentage of caterpillars (unit used = plant with caterpillars) from which parasitoids emerged. Data for B and C were collected between May 1993 and May 1995 in cerrado areas near Brasília. Nine host plant species were examined: Byrsonima spp. (3), Erythroxylum spp. (3), and Qualea spp. (3). The shaded area represents the dry season.
Lepidopteran species and the occurrence of their caterpillars during the year in the cerrado sensu stricto of the
Federal District, Brazil. The period between May to September corresponds to the dry season.
| | | | | | |||||||||
| | | | | | | | | | | | | | |
| Oecophoridae | Cerconota achatina (Zeller, 1855) | | | | | | | | | | | | |
| Hesperiidae | Chiomara punctum (Mabille, 1878) | | | | | | | | | | | ||
| Geometridae | Cyclomia mopsaria (Guenée, 1857) | | | | | | | | | | | ||
| Dalceridae | Dalcerina tijucana (Schaus, 1892) | | | | | | | | | ||||
| Lymantriidae | Eloria subapicalis (Walker, 1855) | | | | | | | | | | | | |
| Arctiidae | Fregela semiluna (Walker, 1854) | | | | | | | | | | | ||
| Oecophoridae | Gonioterma indecora (Zeller, 1854) | | | | | | | | | ||||
| Oecophoridae | Inga haemataula (Meyrick, 1912) | | | | | | | | |||||
| Sphingidae | Isognathus caricae (Linnaeus, 1764) | | | | | | | | | | | | |
| Megalopygidae | Megalopyge albicollis (Walker, 1855) | | | | | | | | |||||
| Limacodidae | Phobetron hipparchia (Cramer, 1777) | | | | | | | ||||||
| Oecophoridae | Stenoma sp. | | | | | | | | | ||||
| Oecophoridae | Stenoma staudingerana (Maassen, 1890) | | | | | | | | | ||||
| Nymphalidae | Siderone marthesia nemesis (Illiger, [1801]) | | | | | | | ||||||
| Urodidae | Urodus sp. | | | ||||||||||
| Oecophoridae | Eonichla sp. | | |||||||||||
| Megalopygidae | Trosia dimas (Cramer, 1775) | | |||||||||||
| Mimallonidae | Mimallonidae sp. | |
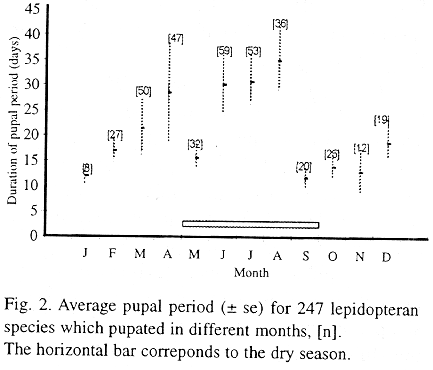
The caterpillar species collected in the field were in various stadia, so complete life cycles are not known. This study was restricted to the presence of caterpillars in the field and pupal duration under laboratory conditions, and no information is available for eggs and adults. The pupal period was variable among individuals and within species (Table 2), and varied according to the time of the year (Fig. 2).
At the beginning of the dry season (Fig. 1a) the insects tended to have shorter pupal periods (Fig. 2), resulting in more generations which coincided with the seasonal peak of caterpillar abundance in the field during this period (Fig. 1b). The duration of the pupal period increased as the dry season progressed and decreased sharply at the transition between the dry and wet seasons. Mean pupal duration, as well as its variance, increased at the end of the wet season (Fig. 2) due, in part, to the presence of species with pupal diapause, such as Trosia dimas and Eonichla sp. (Table 2).
Lepidopteran species and their average pupal period in days, under laboratory conditions.
| | |||||
| Species | | | | | |
| Cerconota achatina (Zeller, 1855) | 131 | 14,0 | 6,23 | 5 | 31 |
| Chiomara punctum (Mabille, 1878) | 13 | 17,8 | 20,63 | 5 | 72 |
| Cyclomia mopsaria Guénée, 1857 | 14 | 11,1 | 5,43 | 4 | 22 |
| Dalcerina tijucana (Schaus, 1892) | 8 | 20,4 | 2,20 | 18 | 24 |
| Eloria subapicalis (Walker, 1855) | 10 | 15,4 | 9,87 | 9 | 41 |
| Fregela semiluna (Walker, 1854) | 43 | 22,0 | 3,95 | 13 | 31 |
| Gonioterma indecora (Zeller, 1854) | 20 | 16,8 | 19,64 | 7 | 96 |
| Inga haemataula (Meyrick, 1912) | 13 | 19,8 | 8,92 | 6 | 34 |
| Isognathus caricae (Linnaeus, 1764) | 8 | 24,0 | 9,87 | 8 | 32 |
| Megalopyge albicollis (Walker, 1855) | 28 | 66,8 | 31,15 | 28 | 144 |
| Phobetron hipparchia (Cramer, 1777) | 9 | 71,2 | 52,07 | 20 | 148 |
| Stenoma sp. | 55 | 98,6 | 54,31 | 7 | 183 |
| Stenoma staudingerana (Maassen, 1890) | 16 | 20,2 | 20,10 | 5 | 81 |
| Siderone marthesia nemesis (Illiger, [1801]) | 5 | 14,6 | 1,95 | 12 | 17 |
| Urodus sp. | 4 | 9,5 | 2,08 | 7 | 12 |
| Eonichla sp. | 1 | 235 | |||
| Trosia dimas (Cramer, 1775) | 1 | 266 | |||
| Mimallonidae sp. | 1 | 351 | |||
Some species have short pupal periods and can occur throughout the year or be restricted to one particular season. For example Siderone marthesia nemesis was found during February and March in four consecutive years (second half of the wet season), feeding on mature leaves of Casearia sylvestris (Flacourtiaceae) (Morais et al. 1996). Its pupal stage lasted 15 days. The adults also occur in the gallery forest (Pinheiro and Ortiz 1992) where they may have more generations. Other species, such as Urodus sp., was found only in November in four consecutive years and always on the same host plant species (Erytroxylum deciduum). Others, for instance Fregela semiluna, Cyclomia mopsaria and Cerconota achatina, have short pupal periods independent of the time of the year (less than 31 days). Cerconota achatina for example, fed on the three Byrsonima species (Andrade et al. 1995) throughout the year and had an average pupal period of 16 days (Table 2). However, the caterpillars collected in the field during the dry season remained as larvae twice as long in the laboratory (54.56 ± 27.2 days, n = 82) as those collected during the wet season (27.5 ± 19.0 days, n = 31; t = 5.08 p < 0.001). These caterpillars did not show any tendency for dormancy and normally continued to feed. Larvae reared on new leaves of B. coccolobifolia produced heavier pupae and bigger adults (I. Andrade pers. com.).
Other species had very long pupal periods during the dry season (Table 2, Fig. 2). In Megalopyge albicollis the duration of the pupal period decreased between early-July and late-August, and the adults emerged synchronously during the wet season in a 20-day span between the end of October and the beginning of November. In Stenoma sp. the long pupal period is variable among individuals and adult emergence is asynchronous, but 65% of the June to August initiated pupae emerged as adults in November.
Relative abundance of parasitoid on caterpillars (unit used was plant with caterpillars) remains low until the beginning of the wet season, in October, when it increases rapidly (Fig. 1c).
Discussion
Relative abundance of caterpillars varied monthly in the cerrado. The highest caterpillar abundance occurred during May and July, and then declined and remained low until mid-April (Fig. 1b). The caterpillar abundance variation in the field was not related to leaf phenology. The higher caterpillar abundance occurred when leaf production was very low (Morais et al. 1995, Price et al. 1995), and most of the existing leaves were old. Soon after (September-October), the majority of cerrado plant species flushed their leaves, followed by the peak of newly expanding leaves in November-December. In spite of all these changes of leaf phenology, caterpillar abundance remained low from August to April.
One partial explanation for the low caterpillar abundance found during the early rainy season could be leaf pubescence. In fact, leaf phenology could be a major factor limiting insect damage on leaves in this cerrado area in the early rainy season (unpublished data), as many cerrado plant species are pubescent with young leaves more hairy than mature ones. Thus, caterpillars that consume pubescent host plants may be rare during September and October when these plants are flushing new leaves.
Janzen (1993) suggests four possible explanations for the low caterpillar abundance registered in the period from four to six weeks after the first rains at Santa Rosa National Park: (1) the delay or spread of oviposition; (2) the time required for eggs to hatch; (3) small species at low density (inconspicuous); and (4) high caterpillar mortality due to predation. Here we suggest elements regarding two other reasons to explain low caterpillar density at the beginning of the rainy season in the cerrado: (5) the accumulated water stress in the late-dry season; and (6) the delay of adult emergence.
In the late dry season there is low precipitation (Fig. 1a), the relative humidity of air is low (often below 20% during warmer hours in September), and the majority of leaves are senescent (poor quality of available food) which, together, probably constrain larval performance and/or increases caterpillar mortality at this time. Even a number of lepidopteran species that normally occurs throughout the year was often absent the field in the months between August and September (Table 1). A similar pattern, with leaf quality reduction and decreased reproductive activity as the dry season progresses was shown for some butterfly species in tropical savannas of Australia (Braby 1995) and in tropical dry forests of Costa Rica (Odendaal 1990).
Some species had very long pupal periods (between 8 and 12 months), suggesting that they may be univoltine and that the pupal stage may function as a resting stage (Table 2). In Costa Rican tropical dry forest a large proportion of moth species spend all or part of the dry season as dormant pupae, and many of them are, indeed, univoltine (Janzen 1987). In the cerrado areas the proportion of univoltine species appears to be low.
The delay in adult emergence at the beginning of the wet season may be related to the unpredictable precipitation in October. Similar patterns were also found for moth species at Santa Rosa National Park in Costa Rica (Janzen 1993). In fact, the onset of rains is variable among years (Fig. 1a), and the species that have long pupal periods during the dry season tend to emerge in November. The longer development period in the dry season may be the result of reduced food quality, water stress, and lower temperature. During October and November the number of caterpillars collected was low and constitutes the first generation after the dry season.
In deciduous dry forest in Costa Rica, Janzen (1987, 1988, 1993) found a peak of caterpillar abundance in the second half of the rainy season. Our results contrast with what was found for Costa Rica. One of the explanations given for this phenomenon, could be the availability of host plant leaves throughout the year in the cerrado. In fact, in the cerrado, many species retain their leaves through the dry season, permitting a broader range of life histories than in the Costa Rican dry forest.
However, the higher caterpillar abundance during the first three months of the dry season (Fig. 1b) is puzzling. Ecological opportunities for phytophagous insects are broad, encompassing such concepts as host plant availability, bioclimatic conditions and enemy-free space. As discussed before, the higher leaf production and most rains occur at a time when caterpillar densities are quite low. Aiello (1992) suggested that the decrease of parasitoids in Panama is a result of the decline in butterfly numbers during the severe dry season and, when humid conditions return, the temporarily enemy-free space (parasitoid) allows the butterfly populations to increase rapidly. Consequently, when parasitoids find themselves in the center of abundance, they also increase and quickly become abundant. The lag of time between the recoveries of hosts and parasitoids make the temporary increase of butterfly populations possible. Similarly, predator and parasitoid activities can play an important role in influencing the numbers of caterpillars encountered in the field and may help keep caterpillar densities down during the wet season in Brazilian cerrado.
It is well known that predators and parasitoids can exert high pressures on caterpillar abundance (Stamp and Casey 1993, Marquis and Whelan 1994). The literature known for cerrado shows that predator abundance is, indeed, highest during the wet season. In Brasília, Polistes nests increase during the rainy season and colonies initiated in the dry season produced fewer pupae than those initiated in the wet season (Ramos and Diniz 1993). Also, bird breeding activity is greatest between September and December (Fernandez 1978, Cavalcanti 1990, Macedo 1992).
Although there are no hard data demonstrating the theory of enemy-free space in the cerrado area, the proportion of caterpillars attacked by parasitoids was lower during dry season than in the early wet season (Fig. 1c). Parasitoid density increases in the early wet season (October), coinciding with the time of lowest caterpillar abundance (Figs. 1b, c). Apparently, the first half of the dry season constitutes the temporary parasitoid enemy-free space.
It is tempting to suggest, as did Janzen (1987, 1988) for Costa Rica, that both parasitoid and predator pressures may regulate the seasonal fluctuations in caterpillar abundance in cerrado area.
Acknowledgements
We thank V. O. Becker and K. S. Brown for the identification of Lepidoptera species; R. Marquis (University of Missouri-St. Louis), P. Price (Arizona State University), W. Benson (University of Campinas), R. Macedo, J. Hay and J. Marinho-Filho, (University of Brasília) for their helpful comments and criticisms on the manuscript; Jair Maia for his help on some figures, R. Henriques (University of Brasília) for allowing us to consult his organized climatic data; and Aurora Bendicho (Camagüey, Cuba) for the spanish abstract. The following students provided field and laboratory assistance in different years: I. Andrade, L. Baumgarten, A. Botelho, D. Coelho, R. Cordeiro, H. Cunha, L. DelClaro, C. Dias, E. Emery, P. Estevanato, M. Lasneaux, R. Malcher, N. Menezes, M. Millhomen, F. Pinto and H. Rocha. The research was supported by Brazilian Research Council (CNPq) grant 520255/95-0. D. Silva, was supported by grant 800083/86-5.
Resumen
Este trabajo se realizó desde 1991 a 1995 en cuatro áreas de cerrado sensu stricto en Brasília, DF, Brasil. El clima de la región es estacional, con las estaciones seca y lluviosa bien marcadas. Nueve especies de plantas hospederas (tres géneros y tres familias) fueron examinadas en 10 800 censos desde 1993 a 1995 y en 15% de ellas se encontraron larvas. Se obtuvieron los adultos de 247 especies de lepidópteros (32 familias). La proporción de plantas con larvas aumentó rapidamente a partir del 15 de abril, permaneciendo alta hasta julio, estación de seca y fue menor al comenzar la estación lluviosa. La duración media del período de pupa varía durante el año (multivoltinas). La baja densidad de larvas al inicio de las lluvias podría deberse al estrés hídrico acumulado durante la estación seca; la defensa química y física de las hojas nuevas; la demora en la emergencia de los adultos y la actividad de depredadores y parasitoides. Probablemente el espacio libre de enemigos durante los tres primeros meses de la época seca favorece la abundancia de larvas en el campo.
References
Aiello, A. 1992. Dry season strategies of two Panamanian butterfly species, Anartia fatima (Nymphalinae) and Pierella luna luna (Satyrinae) (Lepidoptera: Nymphalidae), p. 573-575. In Quintero Arias, D. and Aiello, A. (eds.). Insects of Panama and Mesoamerica: Selected Studies. Oxford University, Oxford. [ Links ]
Andrade, I., I. R. Diniz & H. C. Morais. 1995. A lagarta de Cerconota achatina (Oecophoridae: Stenomatinae): biologia e ocorrência em plantas hospedeiras do gênero Byrsonima (Malpighiaceae). Revta. bras. Zool. 12: 735-741. [ Links ]
Braby, M. F. 1995. Reproductive seasonality in tropical satyrine butterflies: strategies for the dry season. Ecol. Entom. 20: 5-17. [ Links ]
Brown, K. S. & O. H. H. Mielke. 1967. Lepidoptera of the central Brazil plateau. I. Preliminary list of Rhopalocera (continued): Lycaenidae, Pieridae, Papilionidae, Hesperidae. J. Lepid. Soc. 21: 145-168. [ Links ]
Cavalcanti, R. B. 1990. Migrações de aves do cerrado. Anais do IV Encontro Nacional de Anilhadores de Aves (Recife, PE), p. 110-116. [ Links ]
Denlinger, D. L. 1986. Dormancy in tropical insects. Ann. Rev. Entomol. 31: 239-264. [ Links ]
DeVries, P. J. 1987. The butterflies of Costa Rica and their natural history: Papillionidae, Pieridae, Nymphalidae. Princeton University, New York. 327 p. [ Links ]
Diniz, I. R. & H. C. Morais. 1995. Larvas de Lepidoptera e suas plantas hospedeiras em um cerrado de Brasília, DF, Brasil. Revta Bras. Ent. 39: 755-770. [ Links ]
Diniz, I. R. & H. C. Morais. 1997. Lepidopteran caterpillar fauna of cerrado host plants. Biodiver. Conserv. 6: 817-836. [ Links ]
Felfili, J. M. & M. C. Silva. 1993. A comparative study of cerrado (sensustricto) vegetation in central Brazil. J. Trop. Ecol. 9: 277-289. [ Links ]
Felfili I, J. M., M. C. Silva, A. V. Rezende, J. W. B. Machado, B. M. T. Walter, P. E. N. Silva & J. D. HAY. 1993. Analise comparativa da florística e fitossociologia da vegetação arbórea do cerrado sensu stricto na Chapada Pratinha, DF, Brasil. Act. Bot. Bras. 6: 27-46. [ Links ]
Fernandez, R. A. N. 1978. O comportamento alimentar como fator de isolamento ecológico em oito espécies de Tyrannidae (Aves) do Planalto Central. Masters Thesis, University of Brasília, Brasília, DF, Brazil. [ Links ]
Goodland, R. 1971. A physiognomic analysis of "cerrado" vegetation of central Brazil. J. Ecol. 59: 411-419. [ Links ]
Janzen, D. H. 1973. Sweep samples of tropical foliage insects: effects of seasons, vegetation types, elevation, time of day, and insularity. Ecology 54: 687-702. [ Links ]
Janzen, D. H. 1987. How moths pass the dry season in a Costa Rican dry forest. Insect Sci. Applic. 8: 489-500. [ Links ]
Janzen, D. H. 1988. Ecological characterization of a Costa Rican dry forest caterpillar fauna. Biotropica 20: 120-135. [ Links ]
Janzen, D. H. 1993. Caterpillar seasonality in a Costa Rican dry forest, p. 448-477. In N. E. Stamp & T. M. Casey (eds.). Caterpillars - Ecological and evolutionary constraints on foraging. Chapman & Hall, New York. [ Links ]
Jones, R. E. & J. Rienkes. 1987. Reproductive seasonality in the tropical genus Eurema (Lepidoptera: Pieridae). Biotropica 19: 7-16. [ Links ]
Macedo, R. H. 1992. Reproductive patterns and social organization of the communal Guira Cuckoo (Guira guira) in Central Brazil. Auk 109: 786-799. [ Links ]
Mantovani, W. & F. R. Martins. 1988. Variações fenológicas das espécies do cerrado da Reserva Biológica de Mogi Guaçu, Estado de São Paulo. Revta. brasil. Bot. 11: 101-112. [ Links ]
Marquis, R. J. & C. J. Whelan. 1994. Insectivorous birds increase growth of white oak through consumption of leaf-chewing insects. Ecology 75: 2007-2014. [ Links ]
Morais, H. C., I. R. Diniz & L. C. Baumgarten. 1995. Padrões de produção de folhas e sua utilização por larvas de Lepidoptera em um cerrado de Brasília. Revta. brasil. Bot. 18: 165-172. [ Links ]
Morais, H. C., I. R. Diniz & J. R. Silva. 1996. Larvas de Siderone marthesia nemensis (Lepidoptera: Nymphalidae, Charaxinae) em um cerrado de Brasília. Revta. bras. Zool. 13: 351-356. [ Links ]
Odendaal, F. J. 1990. The dry season influences reproductive parameters in female butterflies. Biotropica 22: 100-102. [ Links ]
Pinheiro, C. E. G. & J. V. C. Ortiz. 1992. Communities of fruit-feeding butterflies along a vegetation gradient in central Brazil. J. Biogeography 19: 505-511. [ Links ]
Price, P. W., I. R. Diniz, H. C. Morais & E. S. A. Marques. 1995. The abundance of insect herbivore species in the tropics: high local richness of rare species. Biotropica 27: 468-478. [ Links ]
Ramos, F. A. & I. R. Diniz. 1993. Seasonal cycles, survivorship and growth of colonies of Polistes versicolor (Hymenoptera, Vespidae) in the urban area of Brasília, Brazil. Entomologist 112: 191-200. [ Links ]
Ratter, J. A. 1980. Notes on the vegetation of Fazenda Água Limpa (Brasília, DF, Brazil). Royal Botanic Garden, Edinburgh. 111 p. [ Links ]
Stamp, N. E. & T. M. Casey (eds.). 1993. Caterpillars - Ecological and evolutionary constraints on foraging. Chapman & Hall, New York. 587 p. [ Links ]
Wolda, H. 1988. Insect seasonality: why? Ann. Rev. Ecol. Syst. 19: 1-18. [ Links ]
1 Departamento de Ecologia, Universidade de Brasília, 70910-900 Brasília, DF, Brasil. E-mail: morais@unb.br
2 Departamento de Zoologia, Universidade de Brasília, 70910-900 Brasília, DF, Brasil E-mail: ivone@rudah.com.br