Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.47 n.1-2 San José Jun. 1999
(Hymenoptera: Sphecidae) in southeastern Brazil
Evandro Camillo1 and Antonio D. Brescovit2
Received 15-IV-1998. Corrected 24-XI-1998. Accepted 7-XII-1998.
Abstract
Fifty one nests and 200 cells of Trypoxylon (Trypargilum) lactitarse were obtained from trap-nests (cut bamboo stems) in Santa Carlota Farm (in two habitats: Itaoca Section-IS and Santana Section-SS), Cajuru and on the São Paulo University Campus, Ribeirão Preto (RP), both in the State of São Paulo, Brazil. The prey (spiders) of 18 cells from IS, 38 from SS and 70 from RP were identified. Nesting most frequently occurred during the hot and wet seasons (September to April). T. lactitarse preyed upon representatives of ten spider families. Araneidae (96.6%) (orb-weaver spiders) were the most frequent. Eustala sp.1 was the most frequently collected species in the three habitats (31.6% in IS, 20.1% in SS and 48.7% in RP), followed by Acacesia hamata (19.5%) and Alpaida leucogramma (10%) in IS, by Parawixiaaudax (16%) and A. hamata (15.4%) in SS and by P.audax (17.9%) and Eustala sp.2 (12%) in RP. The sizes of the reproductive niches were significantly different. There was a positive correlation between reproductive niche width and evenness.
Key words
Wasps, Trypoxylon, Araneae, trap-nests, spider prey, niche width, evenness.
Quantitative data concerning prey of predatory animals are usually difficult to obtain. However, solitary wasps deposit their prey in nests that can be easily collected (Rehnberg 1987). Some species that nest in previously existing cavities will use trap-nests which simplifies nest location.
The studies carried out by Hartman (1905), Rau (1928), Muma and Jeffers (1945), Krombein and Evans (1954), Krombein (1956, 1967), Kurczewski (1963), Medler (1967), Matthews and Matthews (1968), Lin (1969), Coville (1979, 1981, 1982), Coville and Coville (1980), OBrien (1982), Hook (1984), Genaro et al. (1989), Camillo et al. (1993), Genaro and Alayón (1994) and Jiménez and Tejas (1994) showed that species or species groups of Trypoxylon have different prey preferences. Differences include the number of families of prey, the proportion of each family, genus and species, as well as the relative proportions of orb-weaving, hunting or wandering spiders.
According to Coville (1987), individuals belonging to the same species may vary in prey "preferences" because they hunt in different areas, exploit spider aggregations or become conditioned to certain types of spiders or to a certain type of hunting behavior. Though some species predominantly collect prey of a single family they still occasionally capture others with distinct habits. The nests of these wasps can provide large numbers of spiders, including species that are rarely collected by man.
Trypoxylon (Trypargilum) lactitarse Saussure, 1867 which occurs from Canada to Argentina (Coville 1981) is a solitary wasp. Female construct linear series of cells that are subdivided by mud partitions, provision each cell with various paralyzed spiders and oviposit on the dorsum of the abdomen of one of the last collected (Coville 1981, Camillo et al. 1993).
This work presents data on prey species collected by T. lactitarse, as well as some parameters of their reproductive niches. These data were obtained from trap-nests placed in three locations in São Paulo State, southeastern Brazil.
Materials and Methods
Study areas: Area 1- Santa Carlota Farm (SCF), Cajuru, SP (21°18-27 S and 47°12-17 W). The vegetation consists of semideciduous mesophyll and riparian forests, "cerrados" (s.l.), "cerradões" (Rizzini,1979) and various types of plantations. Farm altitudes vary from 540 to 944 m. The study sites within this farm, Itaoca Section (=IS) and Santana Section (=SS) (approximately 6 km from each other), were characterized by abandoned orchards surrounded by sugar cane plantations, pasture and "cerrados" (s.l.) with various rural buildings, that were also abandoned. Semideciduous mesophyll and riparian forests were located approximately 300 m from the orchards. Area 2- Campus of São Paulo University, Ribeirão Preto (=RP), SP (21°11 S and 47°13 W) the vegetation of which is formed by small areas of semideciduous forests, "cerradões", grasses, shrubs, ornamental plants, fruit trees and reforestation with arboreous angiosperms. Altitudes vary from 580 to 620 m.
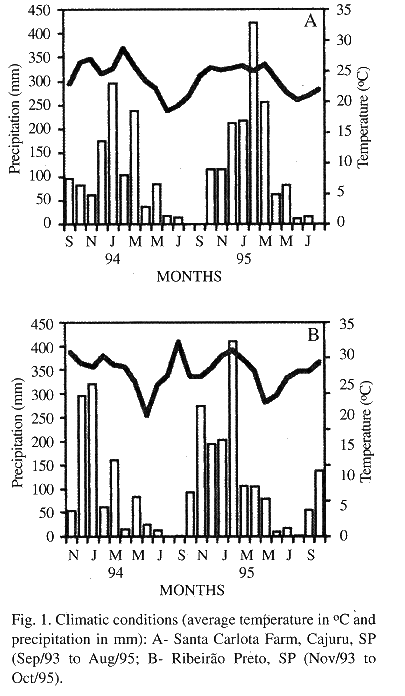
Two distinct seasons are recognized in this region: 1 Cold and dry season (May to August) with monthly average temperatures ranging from 18.5 to 22.2 °C (SCF) and from 17 to 23 °C (RP), precipitation from 0 to 84.4 mm (SCF) and from 0 to 82.9 mm (RP); 2 Hot and wet season (September to April) with monthly average temperatures ranging from 23 to 28.8 °C (SCF) and from 22.4 to 27.2 °C (RP), precipitation from 0 to 422.6 mm (SCF) and from 1.4 to 409.4 mm (RP) (Fig. 1).
Trap-nests: Bamboo stems (B) were used as trap-nests (TN) with a nodal septum closing one of their extremities and internal diameters ranging from 7 to 25 mm and lengths from 70 to 250 mm. These TN (450 in RP, 450 in SS and 200 in IS) were tied together in batches of 6 to 8 units and placed on the shelves of a shelters built at the study locations.
The TN were inspected at least once a month during the period from September/93 to August/95 at SCF and from November/93 to October/95 in RP. During the inspections, which were performed with the help of an otoscope, the nests that had been recently completed or were being provisioned were collected and transported to the laboratory. Each nest was replaced by a new TN. The nests were opened in the laboratory and the prey (spiders) from each cell which contained eggs or recently hatched larvae were removed and preserved in 80% alcohol. At least one immature wasp was left in all of these nests so that it could fully develop and the nesting species be determined. All the wasp and spider specimens are deposited in the Entomology Collection of Biology Department - FFCLRP-USP and part of spiders material was deposited in the arachnological collection of Butantan Institute.
Data analysis: The reproductive niche (Álvarez et al. 1988) width was calculated using the number of prey species as well as the Shannon-Weaver Diversity Index H= - Ph. ln Ph, where ph is the proportion of individuals belonging to the hth species in the total sample (Pielou 1975).
The evenness index was calculated according to Pielou (1966): J= H/ Hmax, where H is the Shannon-Weaver Index and Hmax is the logarithm (ln) of the total number of prey species in the sample. This index varies from zero to one.
The similarity in species composition among the studied habitats was calculated by the Sörensen Similarity Quotient (Sörensen 1948): S.Q. = 2J/ (a+b), where a and b are the numbers of families, genera and species of collected prey in each of the habitats and J is the number of families, genera and species of collected prey common to all samples. The similarity among the studied communities, considering the number of collected spiders of each sampled species, was calculated by the method of percentage similarity (Hanski & Koskela 1977) : PSij = min. (Pih , Pjh), wherePih and Pjh are proportions of the h families, genera or species of collected prey. The "Test for difference between two indices" was used in the statistical analysis of indices (Zar 1984).
Results
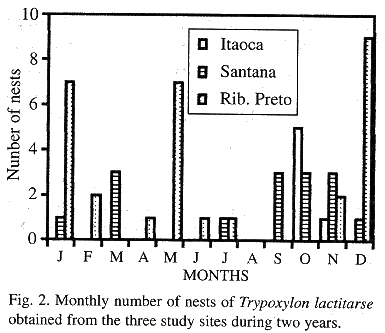
Number of nests, cells and seasonal abundance: 200 cells of T. lactitarse were obtained, 29 were from IS (six nests), 57 from SS (15 nests) and 114 from RP (30 nests), of which prey from 18, 38 and 70 cells, respectively were collected and identified. The nests (11 completed ones and 40 being provisioned) were collected throughout the year, except in the month of August. The greatest nesting frequency took place in the hot and wet season (September to April), when 80.4% of the sampled nests were collected. The months with the greatest nesting frequencies were October in IS, March and from September to November in SS and January, May and December in RP. The occurrence of a large number of nests in May was due to the fact that the temperature in RP, was mild during this month in one of the study years, and decreased only in June (Fig. 2).
Collected prey: T. lactitarse collected individuals from ten families of spiders. Araneidae was the most frequent in all of the studied habitats in number of genera (52.9%), species (72.4%) and individuals (96.6%). Among the other prey families only Anyphaenidae, Araneidae and Salticidae were found at all three sites while Senoculidae was in two (SS and RP). Lycosidae and Tetragnathidae were only collected in IS; Heteropodidae, Theridiidae and Uloboridae only in SS and Trechaleidae only in RP. The fact that Araneidae was the most frequent family in the samples is due to an accentuated preference (97%) by T. lactitarse for collecting species of orb-weaver spiders. Eustala (47.1%) was the most frequent of the genera belonging to the Araneidae family. However, occurs web builder spider on web during the day (Argiope, Eustala, in part, Aculepeira, Leucauge and uloborid species) and web builder spiders off the web which hinding during the day in refuges or in the foliage (the remainder of araneid species listed) (Table 1).
Number of spider juveniles (J), males (M) and females (F) collected by Trypoxylon lactitarse in each one of the studied habitats. IS-Itaoca Section; SS-Santana Section; RP-Ribeirão Preto.
| Species | | | | ||||||
| | | | | | | | | | |
| ANYPHAENIDAE | |||||||||
| Teudis sp. | | | | | | | | | |
| gen. sp. (indeterminate) | | | | | | | | | |
| ARANEIDAE | |||||||||
| Acacesia hamata (Hentz, 1847) | | | | | | | | | |
| Acacesia sp. | | | | | | | | | |
| Aculepeira sp. | | | | | | | | | |
| Alpaida alto Levi, 1988 | | | | | | | | | |
| Alpaida bicornuta (Taczanowski, 1878) | | | | | | | | | |
| Alpaida leucogramma (White, 1841) | | | | | | | | | |
| Alpaida aff. negro | | | | | | | | | |
| Alpaida truncata (Keyserling, 1865) | | | | | | | | | |
| Araneus bogotensis (Keyserling, 1864) | | | | | | | | | |
| Araneus matogrosso Levi, 1991 | | | | | | | | | |
| Araneus sp. 1 | | | | | | | | | |
| Argiope sp. | | | | | | | | | |
| Eustala sp. 1 | | | | | | | | | |
| Eustala sp. 2 | | | | | | | | | |
| Eustala sp. 3 | | | | | | | | | |
| Eustala sp. 4 | | | | | | | | | |
| Eustala sp. 5 | | | | | | | | | |
| Kaira altiventer O.P.-Cambridge, 1889 | | | | | | | | | |
| Larinia t-notata (Tullgren, 1905) | | | | | | | | | |
| Metazigia gregalis (O.P.-Cambridge, 1889) | | | | | | | | | |
| Ocrepeira venustula (Keyserling, 1880) | | | | | | | | | |
| Ocrepeira aff. lapeza | | | | | | | | | |
| Ocrepeira sp. 1 | | | | | | | | | |
| Ocrepeira sp. 2 | | | | | | | | | |
| Ocrepeira sp. 3 | | | | | | | | | |
| Parawixia audax (Blackwall, 1863) | | | | | | | | | |
| Parawixia matiapa Levi, 1992 | | | | | | | | | |
| Parawixia velutina (Taczanowski, 1878) | | | | | | | | | |
| Pozonia sp. | | | | | | | | | |
| Scoloderus sp. | | | | | | | | | |
| Taczanowskia sp. | | | | | | | | | |
| Verrucosa sp. | | | | | | | | | |
| Wagneriana atuna Levi, 1991 | | | | | | | | | |
| Wagneriana eldorado Levi, 1991 | | | | | | | | | |
| Wagneriana aff. atuna | | | | | | | | | |
| Wagneriana aff. silvae | | | | | | | | | |
| Wagneriana sp. 1 | | | | | | | | | |
| Wagneriana sp. 2 | | | | | | | | | |
| Wixia sp. | | | | | | | | | |
| gen. sp. (indeterminate) | | | | | | | | | |
| HETEROPODIDAE | |||||||||
| af. Olios | | | | | | | | | |
| LYCOSIDAE | |||||||||
| gen. sp. (indeterminate) | | | | | | | | | |
| SALTICIDAE | |||||||||
| Chira sp. | | | | | | | | | |
| sp. 1 | | | | | | | | | |
| sp. 2 | | | | | | | | | |
| sp. 3 | | | | | | | | | |
| sp. 4 | | | | | | | | | |
| sp. 5 | | | | | | | | | |
| SENOCULIDAE | |||||||||
| Senoculus sp. | | | | | | | | | |
| TETRAGNATHIDAE | |||||||||
| Leucauge sp. | | | | | | | | | |
| THERIDIIDAE | |||||||||
| Achaearanea sp. | | | | | | | | | |
| gen. sp. (indeterminate) | | | | | | | | | |
| TRECHALEIDAE | |||||||||
| Thaumasia sp. | | | | | | | | | |
| ULOBORIDAE | |||||||||
| af. Zozis | | | | | | | | | |
| Total | | | | | | | | | |
| Grand Total | | | |||||||
Eustala sp1 was the most frequently species of prey in the three habitats (31.6% in IS, 20.1% in SS and 48.7% in RP), followed in IS by Acacesia hamata (Hentz, 1847) (19.5%) and Alpaida leucogramma (White, 1841) (10%), in SS by Parawixiaaudax (Blackwall, 1863) (16%) and A. hamata (15.4%), and in RP by P. audax (17.9%) and Eustala sp.2 (12%). Of the 56 prey species collected, 11 were found in all three study areas, six were common in IS and SS, five in SS and RP and one in IS and RP. Among the other prey four species were only collected in IS, 20 in SS and nine in RP. The distribution of the number of juveniles, males and females collected at all three sites was also determined (Table 1).
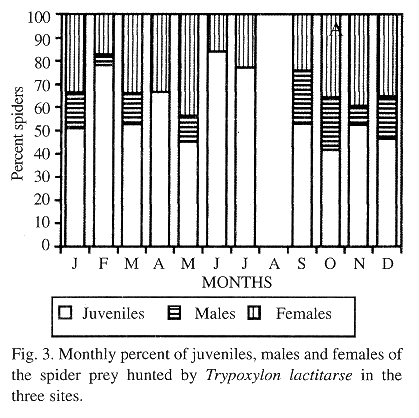
Juveniles, males and females were collected during 8 months of the year while males were not found in April, June or July. The percent of juveniles was always greater than that of females and the latter, in turn, was greater than that of males during all the nesting months. The greatest frequencies of juveniles occurred in the months of February (78.3% of the collected spiders), June (84%) and July (77.1%), of males, in September (22.9%) and October (22.4%), and of females in May (43.8%) and November (39.6%) (Fig. 3). In all locations the juveniles corresponded to 50.3%, females to 34.3% and males to 15.4%.
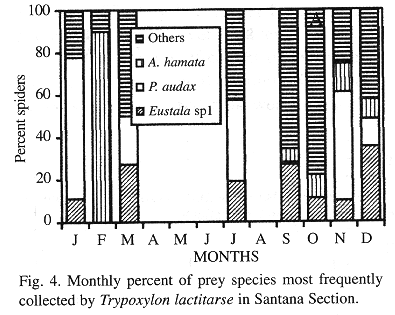
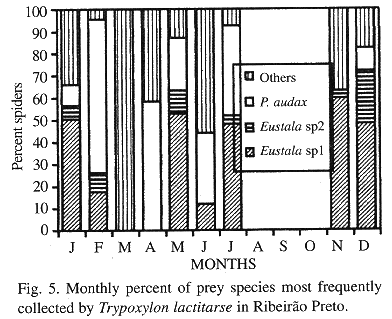
Phenology of the three most frequently collected prey species in SS and RP: In SS, Eustala sp.1 (collected during 7 months) was the most frequent species in March, September and December, P. audax (collected during 6 months) in January, July and November and A. hamata (collected during 5 months) only in February. In October, the preference was divided between Eustala sp.1 and A. hamata (Fig. 4). In RP, Eustala sp.1 (collected during 7 months) was the most frequent species in January, May, July, November and December, P. audax (collected during 8 months) in February, April and June, while Eustala sp.2 (collected during 6 months) was not preferred in any of the months during which nest collection occurred (Fig. 5).
Parameters of the reproductive niche: The monthly reproductive niche width in relation to the number of prey species collected varied from two (February in SS) to 22 (October in IS) while the total niche width varied from 23 (IS) to 42 (SS). The monthly reproductive niche width calculated by the Shannon-Weaver Index (H) varied from 0.33 (February in SS) to 2.46 (October in SS) (Table 2). The correlation between the obtained indices (number of collected species and H) for the monthly niche width was positive and statistically significant (r= 0.80, p<0.05). The total reproductive niche width, H= 2.24 (IS), H= 2.72 (SS) and H= 1.76 (RP) were significantly different (p<0.05).
Reproductive niche and evenness for Trypoxylon lactitarse.
| Months | | | | ||||||||
| | | | | | | | | | |||
| | | | |||||||||
| Jan | | | | | | | | | | ||
| Feb | | | | | | | | | | ||
| Mar | | | | | | | | | | ||
| Apr | | | | | | | | | | ||
| May | | | | | | | | | | ||
| June | | | | | | | | | | ||
| July | | | | | | | | | | ||
| Aug | | | | | | | | | | ||
| Sept | | | | | | | | | | ||
| Oct | | | | | | | | | | ||
| Nov | | | | | | | | | | ||
| Dec | | | | | | | | | | ||
| Total | | | | | | | | | | ||
repeated in the different months. H= Shannon-Weaver index - J= Evenness index
The monthly evenness of the collections varied from 0.47 (February in SS) to 0.89 (June in RP) and the total evenness from 0.53 (RP) to 0.73 (SS) (Table 2). The correlation between the monthly reproductive niche width calculated by the number of collected prey species and evenness (J) was positive and non-significant (r= 0.24, p>0.05) while the correlation between the monthly reproductive niche width calculated by the Shannon-Weaver Index (H) and evenness (J) was positive and significant (r= 0.46, p<0.05).
The greatest similarity values at the family level among the habitats was observed when comparing SS to RP (SQ=0.67), at the genus level, it was greatess when comparing IS to SS (SQ=0.75) and at the species level when comparing IS to RP (SQ=0.56). The percentage similarity was markedly high at the family level and moderate when comparing IS to SS at the genus level (PS=70.4%). The remaining values were relatively low (Table 3).
Similarity Quotients (SQ) and Percentage Similarity (PS) among the habitats in relation to the families, genera and species of prey collected by Trypoxylon lactitarse. IS-Itaoca Section; SS-Santana Section; RP-Ribeirão Preto.
| | | ||||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Discussion
According to Genaro et al. (1989), one of the most important factors in the study of Sphecidae nesting behavior involves the species of captured prey. Many times, not described species and genera are collected, which is important not only from an evolutionary viewpoint, but also from a taxonomic one. This occurs because in order to perform their collections, such wasps, on many occasions, look for prey in places were man does not look.
The female wasps use various techniques to locate their prey: they hunt while flying, hovering in the air near branches, leaves and other plant surfaces and buildings; during flight, they touch surfaces with their antennae and investigate small protuberances and contrasting sites; probably, the contact and the air currents disturb spiders in their shelters or on webs and make them come out to places where they can be more easily hunted (Cross et al. 1975, Coville 1987).
Females of the same species may show preferences for different types of prey (Coville and Coville 1980), which is directly related to the place where they hunt (surface of foliage, among flowers, open vegetation, houses, etc.) (Muma and Jeffers 1945, Coville 1987). Another important factor that influences prey preference is the relative abundance of different spider species, which varies according to the season and from one region to another, especially in regions of temperate climate. Different preferences may also be related to the fact that wasps can exploit aggregations of spiders, become specialists in capturing certain types of prey or present conditioned hunting behaviors (Coville 1987). The proportion and composition of species indicate that prey selection does not follow an automatic and inflexible behavior pattern, but rather an adaptive response to their abundance and distribution (Genaro and Alayón 1994).
The prey collected by T. lactitarse in the three studied sites were mostly spiders belonging to the Araneidae family (96.6%), which according to Horner and Klein (1979), reflects the diversity of habitats occupied by such group of spiders. Owing to this fact, such species almost exclusively (97%) collected orb-weaver spiders. From these results we can suppose that the prey families which occurred in the samples in small percents could have been occasionally collected. If the majority of Araneidae species captured by T. lactitarse are web builder spider that hide of out of web, in refuges or in the foliage during the day and according Levi (1977) Eustala present species which rest on a dead branch during the day, we can suggests that Eustala sp.1 and Eustala sp.2 can be web builder spider off the web. Thus 97.6% of all Araneidae captured can belong to this group.
Tables 4 and 5, in which the species are arranged according to Coville (1982), clearly show differences in spiders captured by some species of the subgenus Trypargilum. Those belonging to the nitidum group, except the species of the spinosum complex and T. xanthandrum Richards, 1934 (fugax complex), preyed preferentially orb-weaver spiders and, among them, the species of the Araneidae family were the most common. Only T. tenoctitlan Richards, 1934 and T. subimpressum F. Smith, 1856 collected less than 50% of individuals of this family. The spinosum complex species collected more prey of the Salticidae and Thomisidae families (hunting or wandering spiders); however, T. saussurei Rohwer, 1912 and T. texense Saussure, 1867 also collected Araneidae. The collection of only this type of prey was also observed in T. xanthandrum, which exclusively (100%) captured individuals of the Senoculidae family. The species of the albitarse and superbum groups markedly captured prey from a single family, Araneidae and Salticidae, respectively.
Percents of spider families collected by Trypoxylon species of the nitidum Group.
| | Complex | | | |||||||
| Spider prey | | | | | | | | | | |
| | | | | | | | | | | |
| SNAREBUILDERS | ||||||||||
| Araneidae | | | | | | | | | | |
| Theridiidae | | | | | | | | | | |
| Tetragnathidae | | | | | | | | | | |
| Linyphiidae | | | | | | | | | | |
| Pholcidae | | | | | | | | | | |
| Dictynidae | | | | | | | | | | |
| Uloboridae | | | | | | | | | | |
| INTERMEDIATES | ||||||||||
| Mimetidae | | | | | | | | | | |
| WANDERING | ||||||||||
| SPIDERS | ||||||||||
| Anyphaenidae | | | | | | | | | | |
| Clubionidae | | | | | | | | | | |
| Lycosidae | | | | | | | | | | |
| Oxyopidae | | | | | | | | | | |
| Pisauridae | | | | | | | | | | |
| Salticidae | | | | | | | | | | |
| Thomisidae | | | | | | | | | | |
| Mismenidae | | | | | | | | | | |
| Heteropodidae | | | | | | | | | | |
| Senoculidae | | | | | | | | | | |
| REFERENCESb | | | | | | | | | | |
Percents of spider families collected by Trypoxylon species of the nitidum, albitarse and superbum Groups.
| | | | ||||||||
| Spider prey | | | | Complex | | |||||
| | | talis | | tarse | hoferi | num | | verdae | bum | |
| | | | | | | | | | | |
| SNAREBUILDERS | ||||||||||
| Araneidae | | | | | | | | | | |
| Theridiidae | | | | | | | | | | |
| Tetragnathidae | | | | | | | | | | |
| Linyphiidae | | | | | | | | | | |
| Uloboridae | | | | | | | | | | |
| INTERMEDIATES | ||||||||||
| Mimetidae | | | | | | | | | | |
| WANDERING | ||||||||||
| SPIDERS | ||||||||||
| Anyphaenidae | | | | | | | | | | |
| Clubionidae | | | | | | | | | | |
| Pisauridae | | | | | | | | | | |
| Salticidae | | | | | | | | | | |
| Thomisidae | | | | | | | | | | |
| Trechaleidae | | | | | | | | | | |
| Scytodidae | | | | | | | | | | |
| Heteropodidae | | | | | | | | | | |
| Oxyopidae | | | | | | | | | | |
| Ctenidae | | | | | | | | | | |
| REFERENCESb | | | | | | | | | | |
The low proportion of adult male spiders in provisions of T. lactitarse could be the result of the behavioral pattern found in Araneidae (Rehnberg 1987). Males had a smaller time of life than females and are found only during the reproduction season (Jiménez and Tejas 1994). When they are sexually mature, females continue occupying webs and feeding themselves while males interrupt feeding and start looking for females (Gertsch 1949). Thus, females become more vulnerable prey as well as better in terms of energy since individuals of several species have larger abdomens than those of males (Rehnberg 1987).
The great preference for juveniles observed in this work is probably related to the fact that, in some spider species, after young individuals emerge, large numbers of them remain near the egg sacs (Jones 1983). They make their own webs to capture their own food, although such webs are still small. As Trypoxylon transport their prey during flight, the preference for collecting young individuals probably depends upon the relative abundance of different spiders sized in the field (Coville 1979, 1987) because the body size of juveniles which are similar to males (7.7 to 9.6 mm) but smaller than females (10.0 to 20.8 mm) (Levi 1992). This fact was observed in this work since T. lactitarse usually preyed upon juveniles (99%) of P. audax.
Collection of large percentage of juveniles was also observed for T. texense (75%) (Kurczewski 1963), T. arizonense Fox, 1891 (70%) (Matthews and Matthews 1968), T. politum Say, 1837 (88%) (Barber and Matthews 1979), T. orizabense Richards, 1934 (76.5%) (Coville 1980), T. tenoctitlan (76.2%) (Coville and Coville 1980), T. nitidum schultessi Richards, 1936 (73%), T. lactitarse (53.8%) and T. saussurei (75%) (Coville 1981), T. monteverdae Coville 1982 (100%) (Coville 1982, Brockmann 1992), T. occidentalis Coville 1982 (97%) (Coville 1982), T. xanthandrum (95.6%) (Coville and Griswold 1983), T. superbum F. Smith, 1873 (35.5%) (Coville and Griswold 1984), T. subimpressum (34% young individuals and 39% sub-adults) (Genaro and Alayón 1994) and T. tridentatum tridentatum Packard, 1867 (68.9%) (Jiménez and Tejas 1994).
Rehnberg (1987) observed that the preference (60%) of T. politum for juveniles occurred only at the beginning of summer (Georgia-USA) and decreased to <5% at the end of the season. This large variation was not observed in this work since T. lactitarse preferably collected juveniles throughout the year. These results probably indicate the occurrence of more than one generation per year in some of the sampled prey species, which must also be related to the fact that T. lactitarse has at least four generations per year in our region (Camillo et al. 1993).
Although numerous species of Trypoxylon (Trypargilum) collected prey of the Eustala genus, an accentuated preference, as observed in this work has only been observed for T. politum (Cross et al. 1975) and T. monteverdae (Coville 1982, Brockmann 1992) in the United States and Costa Rica, respectively.
The reproductive niche size of T. lactitarse in SS (H= 2.72) and RP (H= 1.76) were similar to those observed (as diversity) for T. subimpressum (Genaro and Alayón 1994) at three locations in Cuba (H= 2.6, H= 2.5 e H= 1.7). At a fourth location, the size of the reproductive niche was smaller (H= 1.3) than those observed in our study.
Acknowledgements
We are especially grateful to the owners of Santa Carlota Farm, Cajuru, SP, for allowing us free access to their land. We thank Carlos A. Garófalo for his comments and suggestions and José Carlos Serrano for technical help.
References
Álvarez, V.B., J.A. Genaro & C.S.S. Alonso. 1988. Nuevas considerations acerca del nicho ecológico. Ciencias Biologicas 19/20: 3-8. [ Links ]
Barber, M.C. & R.W. Matthews. 1979. Utilization of trap nests by the pipe-organ mud-dauber, Trypargilum politum (Hymenoptera: Sphecidae). Ann. Entomol. Soc. Am. 72: 260-262. [ Links ]
Brockmann, H.J. 1992. Male behavior, courtship and nesting in Trypoxylon (Trypargilum) monteverdae (Hymenoptera: Sphecidae). J. Kans. Entomol. Soc. 65: 66-84. [ Links ]
Camillo, E., C.A. Garófalo, G. Muccillo & J.C. Serrano. 1993. Biological observations on Trypoxylon (Trypargilum) lactitarse Saussure in southeastern Brazil (Hymenoptera: Sphecidae). Rev. Bras. Entomol. 37: 769-778. [ Links ]
Camillo, E., C.A. Garófalo & J.C. Serrano. 1994. Observações sobre a biologia de Trypoxylon (Trypargilum) rogenhoferi Kohl (Hymenoptera: Sphecidae). An. Soc. Entomol. Brasil 23: 299-310. [ Links ]
Coville, R.E. 1979. Biological observations on Trypoxylon (Trypargilum) orizabense Richards in Arizona (Hymenoptera: Sphecidae). J. Kans. Entomol. Soc. 52: 613-620. [ Links ]
Coville, R.E. 1981. Biological observations on three Trypoxylon wasps in the subgenus Trypargilum from Costa Rica: T. nitidum schultessi, T. saussurei and T. lactitarse (Hymenoptera: Sphecidae). Pan-Pacif. Ent. 57: 332-340. [ Links ]
Coville, R.E. 1982. Wasps of the genus Trypoxylon subgenus Trypargilum in North America. University of California, Berkeley, California 147 p. [ Links ]
Coville, R.E 1987. Spider-hunting sphecid wasps, p. 309-318. In W. Nintwig (ed.). Ecophysiology of spiders. Springer, Berlin. 448 p. [ Links ]
Coville, R.E. & P.L. Coville. 1980. Nesting biology and male behavior of Trypoxylon (Trypargilum) tenoctitlan in Costa Rica (Hymenoptera: Sphecidae). Ann. Entomol. Soc. Am. 73: 110-119. [ Links ]
Coville, R.E & C. Griswold. 1983. Nesting biology of Trypoxylon xanthandrum in Costa Rica with observations on its spider prey (Hymenoptera: Sphecidae; Araneae: Senoculidae). J. Kans. Entomol. Soc. 56: 205-216. [ Links ]
Coville, R.E. & C. Griswold. 1984. Biology of Trypoxylon (Trypargilum) superbum (Hymenoptera: Sphecidae), a spider-hunting wasp with extended guarding of the brood by males. J. Kans. Entomol. Soc. 57: 365-376. [ Links ]
Cross, E.A., M.G. Stith & T.R. Bauman. 1975. Bionomics of the organ-pipe mud-dauber, Trypoxylon politum (Hymenoptera: Sphecidae). Ann. Entomol. Soc. Am. 68: 901-916. [ Links ]
Garcia,M.V.B. & J. Adis. 1995. Comportamento de nidificação de Trypoxylon (Trypargilum) rogenhoferi Kohl (Hymenoptera, Sphecidae) em uma floresta inundável de várzea na Amazônia Central. Amazoniana 13: 259-282. [ Links ]
Genaro, J.A., C.S. Sanchez & G. Alayón. 1989. Notas sobre la conducta de nidificación de Trypoxylon (Trypargilum) subimpressum Smith (Hymenoptera: Sphecidae). Caribbean J. Sci. 25: 228-229. [ Links ]
Genaro, J.A. & G. Alayón. 1994. Las presas (Araneae) de Trypoxylon (Trypargilum) subimpressum (Hymenoptera: Sphecidae) en Cuba. Rev. Biol. Trop. 42: 353-356. [ Links ]
Gertsch, W.J. 1949. American spiders. D. Van Nostrand Co., Inc., Princeton, New Jersey. [ Links ]
Hanski, I. & H. Koskela. 1977. Niche relations among dung inhabiting beetles. Oecologia 28: 203-231. [ Links ]
Hartman, C. 1905. Observations on the habits of some solitary wasps of Texas. Bull. Univ. Texas 65: 1-72. [ Links ]
Hook, A.W. 1984. Notes on the nesting and mating behavior of Trypoxylon (Trypargilum) spinosum (Hymenoptera: Sphecidae). J. Kans. Entomol. Soc. 57: 534-535. [ Links ]
Horner, N.V. & J.H. Klein Jr. 1979. Spider prey of two mud dauber wasp species in Comanche Country Oklahoma (Hymenoptera: Sphecidae). Environ. Entomol. 8: 30-31. [ Links ]
Jiménez, M.L. & A. Tejas. 1994. Las arañas presa de la avispa lodera Trypoxylon (Trypargilum) tridentatum tridentatum en Baja California Sur, Mexico. Southwest. Entomol. 19: 173-180. [ Links ]
Jones, D. 1983. A guide to spiders of Britain and northern Europe. Hamlyn, London. 320 p. [ Links ]
Krombein, K.V. 1956. Biological and taxonomic notes on the wasps of Lost River State Park, West Virginia, with additions to the faunal list (Hymenoptera, Aculeata). Proc. Ent. Soc. Wash. 58: 153-161. [ Links ]
Krombein, K.V. 1967. Trap-nesting wasps and bees. Life histories, and associates. Smithsonian Institution, Washington, D.C. 570 p. [ Links ]
Krombein, K.V. & H.E. Evans. 1954. A list of wasps collected in Florida, March 29 to April 5, 1953, with biological annotations (Hymenoptera, Aculeata). Proc. Ent. Soc. Wash. 56: 225-236. [ Links ]
Kurczewski, F.E. 1963. A first Florida record and note on the nesting of Trypoxylon (Trypargilum) texense Saussure (Hymenoptera: Sphecidae). Fla. Entomol. 46: 243-245. [ Links ]
Levi, H.W. 1977. The american orb-weaver genera Cyclosa, Metazygia and Eustala north of Mexico (Araneae, Araneidae). Bull. Mus. Comp. Zool. 148: 61-127. [ Links ]
Levi, H.W. 1992. Spiders of the orb-weaver genus Parawixia in America (Araneae: Araneidae). Bull. Mus. Comp. Zool. 153: 1-46. [ Links ]
Lin, C.S. 1969. Biology of Trypoxylon spinosum, with notes on T. texense and T. politum at Lake Texoma (Hymenoptera: Sphecidae: Trypoxyloninae). Wasmann J. Biol. 27: 125-133. [ Links ]
Matthews, R.W. & J.R. Matthews. 1968. A note on Trypargilum arizonense in trap nests from Arizona, with a review of prey preferences and cocoon structure in the genus (Hymenoptera: Sphecidae). Psyche 75: 285-293. [ Links ]
Medler, J.T. 1967. Biology of Trypoxylon in trap nests in Wisconsin (Hymenoptera: Sphecidae). Am. Midl. Nat. 78: 344-358. [ Links ]
Muma, M.H. & W.F. Jeffers. 1945. Studies on the spider prey of several mud-dauber wasps. Ann. Entomol. Soc. Am. 38: 245-255. [ Links ]
OBrien, M.F. 1982. Trypargilum tridentatum (Packard) in trap nests in Oregon (Hymenoptera: Sphecidae: Trypoxylinae). Pan-Pacif. Ent. 58: 288-290. [ Links ]
Pielou, E.C. 1966. An introduction to Mathematical Ecology. John Wiley, New York, 286 p. [ Links ]
Pielou, E.C. 1975. Ecological diversity. John Wiley, New York, 165 p. [ Links ]
Rau, P. 1928. Field studies in the behavior of the non-social wasps. Trans. Acad. Sci. St. Louis 25: 325-362. [ Links ]
Rau, P. 1943. The nesting habits of certain sphecid wasps of Mexico, with notes on their parasites. Ann. Entomol. Soc. Am. 36: 647-653. [ Links ]
Rau, P. 1944. The prey and hunting habits of the wasp, Trypoxylon politum Say. Entomol. News 55: 9-10. [ Links ]
Rehnberg, B.G. 1987. Selection of spider prey by Trypoxylon politum (Say) (Hymenoptera: Sphecidae). Can. Ent. 119: 189-194. [ Links ]
Rizzini, C.D. 1979. Tratado de fitogeografia do Brasil, Vol. 2. São Paulo, Hucitec Ltda., Edusp. 374 p. [ Links ]
Sörensen, T. 1948. A method of establishing group of equal amplitude in plant sociobiology based on similarity of species content and its application to analyses of the vegetation on Danish commons. Biol. Skr. 5: 1-34. [ Links ]
Zar, J.H. 1984. Biostatistical analysis. Prentice-Hall, Inglewood Cliffs, New Jersey, 718 p. [ Links ]
1 Departamento de Biologia, Faculdade de Filosofia Ciências e Letras de Ribeirão Preto-USP, 14040-901, Ribeirão Preto, SP, Brasil . E-mail: ecamillo@ffclrp.usp.br
2 Laboratório de Artrópodes Peçonhentos, Instituto Butantan, Av. Vital Brasil 1500, 05503-900, São Paulo, SP, Brasil.