Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.46 n.3 San José Sep. 1998
Abstract
Boophilus microplus infestation is one of the most serious limitations to cattle industry in tropical regions, even though bovines show natural resistance to ticks. This resistance was evaluated in Cross-bred Carora Bulls (CCB) a tropicalized dairy breed from Venezuela. Seven CCB were experimentally infested with B. microplus larvae, "Mozo" strain, they were considered tick-naive because they had never been infested with ticks. The mean inoculum size applied on each bull was 6 477 larvae. After life cycle was completed adult female body weight (BW), egg mass weight (EW), egg hatching rate (%EH), and reproductive index (RI) were recorded. Results revealed a high variability in the levels of resistance to B. microplus. Thus, one animal showed greater resistance (Dunnett, p< 0.05) for the analyzed parameters in contrast with three non-resistant bulls. The others had moderate resistance. The trait "resistance" should be included togheter with other traits often used in genetic selection of cattle.
Key words
Boophilus microplus, Carora breed cattle, resistance to ticks, natural resistance.
Ticks, in particular Boophilus microplus Canestrini, 1887, are the most important ectoparasites of cattle in the tropics and subtropical regions, not only due to the direct damage these arthropods cause on cattle herds, but also for the transmission of diseases such as babesiosis, anaplasmosis, theileriosis and cowdriosis. On this century, tick control has been mainly carried out by chemical methods applying specific acaricides on parasitized cattle. Nonetheless, tick control by chemicals is expensive, most of these acaricides are harmful for the environment, they are often detected in milk and meat of treated animals, and its excessive and empirical use has led to the development of tick resistance to these chemicals (Nuñez et al. 1982). Field reports from all continents are indicating that tick resistance to acaricides is alarmingly increasing, therefore, the main objective of chemical control is not been achieved in the tropics nor in other regions (Solomon 1983, Roush 1993). In Venezuela, the acaricide resistance situation is also similar, and therapeutic failures after acaricide treatments are well documented (Coronado & Mujica 1997). Thus, other effective methods in tick control must be used if we want to fight successfully against these ectoparasites, and simultaneously to keep an acceptable level of milk and/or meat production in the tropics. One of these methods is the use of breeds of cattle which are either totally resistant or have some degree of resistance to ticks (Frisch 1981, FAO 1990).
First attempts to evaluate natural resistance of cattle against B. microplus were made in Brazil (Villares 1941), using Bos indicus cattle to promote resistance against B. microplus. Later studies showed that cattle resistance to ticks was due to the mounting of a protective immune response against ticks by bovine hosts, and this response was heritable for the cattle progeny (Roberts 1968, Roberts & Kerr 1976). In addition, it is well known that resistance to B. microplus varies, not only between individual
bovines, but also from breed to breed, even in B. indicus (Zebu cattle) as in Bos taurus (European cattle) herds (Francis & Little 1964, Roberts 1968). In general, pure or cross-bred Zebu cattle is worldwide acknowledged as more resistant to B. microplus and other ticks than European cattle (B. taurus). The methodology for studying the levels of host resistance to ixodid ticks is well known (Wharton & Norris 1980, Wikel 1996). Briefly, host natural or acquired resistance to ticks by a cattle breed is determined after assessing several ticks feeding and fertility parameters, i.e., reduced engorgment weight, length of feeding period, reduction of tick egg production, reduced percentage of egg hatching, and egg and larvae mortality (Wikel 1996). The application of this methodology and later the genetic selection of those tick resistant cattle led in several countries, i.e. Australia, Brazil and some regions in Africa, to develop tick-resistant cattle breeds after cross-breeding B. taurus x B. indicus. Among these cattle breeds developed in the tropics are the Sahiwal, Belmont Red, Bradford, Bonsmara, NDama and Boran (De Castro & Newson 1993). Australia has been the most successful country in raising breeds of tick-resistant cattle with significant productivity and with high adaptability to the tropical environment. In Australia this success is mainly due to the deep knowledge they have on ecology and population dynamics of ticks and because in this continental island the individual cattle resistance to ticks has been quantified (Wharton & Norris 1980, De Castro & Newson 1993).
In Venezuela, Lara State, Carora region, a tropicalized dairy breed is in the process of developement since 1920 which has been named Carora, and it was officially accepted as a breed in 1989 by the Venezuelan Agriculture and Husbandry Ministry. This breed is the product of empirical genetic crosses among Brown Swiss cattle, indigenous Creole bovines, and Bos indicus in a minor degree (Herrera 1960, Meléndez & Forlano 1996). The Carora breed cattle shows an average milk yield /cow, per day, of 12 lts., and it shows some levels of resistance to tropical diseases (Herrera 1960).
In 1993 a preliminary assay was conducted in order to evaluate the levels of tick resistance by Carora breed heifers and cows naturally infected with B. microplus. We found a B. microplus reproductive index (RI) of 38.16% for Carora cattle whereas Holstein cows showed a RI of 45.48% (Cerutti et al. 1995). As a continuation of this previous assay this work was undertaken with the main objective of assessing the levels of natural resistance to B. microplus in seven (7) tick-naive cross-bred Carora bulls (CCB) which were experimentally infected with larvae of this tick.
Materials and Methods
Experimental animals: Seven (7) CCB with mean age of 31 months (range: 26 – 45 months) were used in order to evaluate the degree of natural resistance in this tropicalized breed against B. microplus. These bulls had been kept in boxes from birth to adult without contact with ticks, thus we considered them as tick-naive bovines. When the experiment started bulls were located in boxes at the Carora Artificial Insemination Center (CIAC) since they had been genetically selected as semen donors for an AI program. Bulls had a mean body weight of 623 Kg (range: 538-774 Kg) at the beginning of this trial, and they had received a last mosquicide treatment 35 days ago. Bulls were housed in individual roofed boxes and their body temperature was daily checked during the whole biological cycle of the tick (21 days). In addition, none insecticide was applied at CIAC during this period and movement of other bovines was tight controled.
Tick infestation: The experimental group of bulls was infested with B. microplus larvae, "Mozo" strain, which was kindly supplied by Dr. A. Nari, Instituto Miguel Rubino, Montevideo, Uruguay. This strain has been kept at our laboratory through passages in tick-susceptible calves. Each bull was infested with a mean inoculum of 6 477 larvae which were 21 day old for the time ticks were applied along the dorsal line of each bovine. Seventeen (17) days post infestation (PI) partially fed female B. microplus were counted on each bull; the process of counting was performed only on the right side of each bovine following a known technique (OKelly & Seifert 1969). Later, on days 21, 22 and 23 PI all adult engorged female (EF) B. microplus was collected from their hosts (tick standard lenght used: 4.5 - 8.0 mm) and brought to the laboratory facilities.
Incubation of B. microplus: Each collected tick was counted, water washed, dried and weighted, next a random sample (1.6% - 39.5%) of these ticks (Table 2) were adhered at masking tape and placed inside an incubator at 27°C ± l°C and 90% relative humidity (RH) (Dalgliesh & Stewart 1982). At the end of the oviposition period each normal egg mass (eggs with an oily and shinning shell plus an amber colour) was collected, weighted and placed in vials sealed with cotton plugs. Vials were properly identified and placed inside the incubator until the process of larvae hatching ocurred. A total of four (4) parameters related to ticks feeding and fertility were recorded and later statistically analyzed. These parameters were: adult female tick body weight (BW), egg mass weight (EW), egg hatching rate (%EH), and the reproductive index (RI) (RI= EW/BW x %EH).
Statistical analysis: The four recorded parameters were described by their means (X), variance and standard error. Next, a multiple comparative study of their variances was conducted and the significant differences detected were analyzed using the Dunnetts test (SAS: Statistical Analysis System) which led to compare Fc vs Ft (p< 0.0001).
Results
Tick parameters: Boophilus microplus larvae completed their life cycle in all infested bulls after 21 ± 2 days PI and no clinical signs of tick-borne diseases were seen in these bovines during the evaluation period (mean rectal temperature: 38.5°C; range: 38.0°C - 39.6°C). The population of partially fed female B. microplus counted on day 17 PI on each bull showed great variations (range: 63 - 670 ticks) (Table 1). The size of each larvae inoculum applied to each bovine plus the percentage of EF B. microplus recovered are also shown in Table 1 (Note: in tables 1 and 2 each row of data belongs to each individual bull).
5.300 373 32.64
Rows: individual bulls
PI: post infestation
EF: engorged females
A total of 307 EF B. microplus were placed in incubation, these ticks had a mean BW of 213.1 mg (range: 179 - 233 mg) (Table 2), and their pre-oviposition period varied between 2 to 4 days. After 2 weeks of oviposition egg masses were weighted and variations were detected in this parameter (Table 2). The embrionary period lasted 28 days without noticeable changes. The mean %EH was calculated after stereomicroscopic countings of a mixture of hatched larvae plus unhatched eggs from each EF incubated tick, and this parameter ranged from 14% to 50% (Table 2). Due to these evident variations in the %EH, the RI for each EF group of ticks also showed significant variations among the 7 bulls (Table 2).
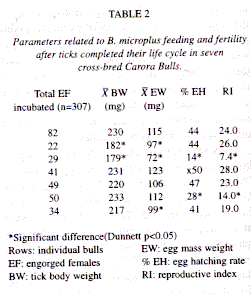
Resistance of CCB to ticks: The comparative analysis of variances of BW, EW, %EH and RI of collected EF B. microplus showed significant differences (p < 0.001) between the means of these parameters. Nevertheless, when the Dunnetts test was applied, significant differences (Dunnett p <0.05) were only shown between the mean BW of ticks collected from bulls 2 and 3. In addition, no significant differences (Dunnett p > 0.05) were detected for the mean BW of ticks from other bulls (Table 2). Applying the same test, significant differences (Dunnett p< 0.05) were observed for the parameter EW of ticks collected from bulls 2, 3, and 7, whereas only for bulls 3 and 6 were detected significant differences (Dunnett p< 0.05) for the parameter %EH (Table 2). Finally, the RI only showed significant differences (Dunnett p< 0.05) for bulls 3 and 6 (Table 2).
In summary, CCB 3 showed the highest level of resistance since it yielded EF ticks with the lowest mean BW, therefore its EF B. microplus laid minor egg masses, with the lowest %EH (14%) and the lowest RI (7.4) (Table 2). In contrast, bulls 2, 6 and 7 showed a medium level of resistance whereas the other three bulls were considered as non-resistant on the basis of comparing their studied tick parameters against those of bull 3.
Discussion
This study has shown that CCB experimentally infested with B. microplus showed great variability in their natural resistance to this tick, thus one bull out of seven was statistically detected as tick-resistant after experimentally infested, three were non-resistant, and three showed moderate tick-resistant. This resistance was evaluated in the lab after a multiple comparative analysis of four EF B. microplus parameters. As it is well known resistance to B. microplus varies between individual cattle, from breed to breed, and among species of cattle. i.e., Bos indicus is more resistant to ticks than B. taurus (De Castro & Newson 1993). These statements are in agreement with the finding that the character "tick resistance" is inheritable by cattle offspring almost in direct proportion to the percentage of Zebu cattle present in the dam or sire (Arteche 1987). Besides, this character is rather easy to detect and measure in the lab since the genetic composition of bovine hosts has a strong influence on the life cycle of B. microplus or other ticks (Barriga et al. 1993). On the basis of our results, we consider that this "strong influence" was detected and evaluated in CCB due to its genetic origin.
Carora breed cattle is the product of genetic crosses among Creole cattle, Bos taurus, and Bos indicus cattle, consequently any Carora bovine classified as tick-resistant should have a greater proportion of B. indicus genes, those classified as moderate tick-resistant should probably have an even proportion of B. indicus and B. taurus genes, and finally those considered as non-tick-resistant should have a predominance of B. taurus genes.
The great variability detected for B. microplus resistance in CCB is important in particular in a tropical breed in the process of genetic consolidation, since cattle owners and field technicians should start genetic programs selecting Carora heifers and bulls, not only on the basis of phenotype, milk production and breeding data, but also including the factor levels of resistance to B. microplus. In general, it can be stated that the character tick resistance must be included in a genetic program of cattle breeding because it meets these 4 basic conditions: 1) it has a high genetic variability, 2) it is inheritable, 3) it is economically important and 4) it is rather easy to calculate. The levels of B. microplus resistance were evaluated through four parameters related to feeding and fertility of this tick, and no relying only on the EF tick burdens as it is often tested and recently questioned (Barriga et al. 1995). Finally, we believe that an effective program of tick control in the tropics can be accomplished only using the basic principles of integrated pest management, and in particular using tick-resistant cattle.
Acknowledgements
To the Officers of Carora Breed Cattlemen Association (ASOCRICA), Carora, Venezuela, to the personnel of CIAC, and to Deicy Mendoza and María G. Ramírez, Veterinary Medicine students, for their technical assistance.
References
Arteche, C. P. 1987. Consideraciones prácticas sobre el empleo de razas resistentes de vacunos parala lucha contra las garrapatas en América Latina. Informe Final. Consulta de expertos sobre erradicación de garrapatas, con especial referencia a América Latina. FAO. México, 12 p. [ Links ]
Barriga, O., A. Da Silva & J. Azevedo. 1993. Inhibition and recovery of tick functions in cattle repeateadly infested with Boophilus microplus. J. Parasitol. 79: 710-715. [ Links ]
Barriga, O., A. Da Silva & J. Azevedo. 1995. Relationships and influences between Boophilus microplus characteristics in tick-naive or repeatedly infested cattle. Vet. Parasitol. 56: 225-238. [ Links ]
Cerutti, F., R. Rizzi, R. D. Meléndez & A. Coronado. 1995. Resistenza a Boophilus microplus nella razza bovina Carora. XI Congresso Nazionale Associazione Scientifica di Produzione Animale (ASPA). Grado, Gorizia, Italy. 2 p. [ Links ]
Coronado, A. & F. Mujica. 1997. Resistencia a acaricidas en Boophilus microplus en Venezuela. Gac. Cien. Vet. 3: 5-13. [ Links ]
Dalgliesh, R. J. & N. P. Stewart. 1982. Some effects of time, temperature and feeding on infection rates with Babesia bovis and Babesia bigemina in Boophilus microplus larvae. Int. J. Parasitol. 12: 323-326. [ Links ]
De Castro, J. J. & R. M. Newson. 1993. Host resistance in cattle tick control. Parasitol. Today 9: 13-17. [ Links ]
FAO. 1990. Report of the FAO Expert Consultation on revision of strategies for the control of ticks and tick-borne diseases. Parassitologia (Roma). 32: 3-12. [ Links ]
Francis, J. & D. A. Little. 1964. Resistance of Drougtmaster cattle to tick infestation and babesiosis. Aust. Vet. J. 40: 247-253. [ Links ]
Frisch, J. E. 1981. Factors affecting the resistance to ecto- and endoparasites of cattle in tropical areas and the implications for selection, p. 17-32. In IAEA (ed.). Isotopes and radiation in Parasitology IV. Panel Proceeding Series. Pub. 572, Vienna. [ Links ]
Herrera, D. J. 1960. El mestizo Pardo Suizo Caroreño como base para un futuro tipo de ganado. Rev. Vet. Venezol. 9: 343-388. [ Links ]
Meléndez, R. D. & M. Forlano. 1996. Incidence and intensity of Babesia spp. sporokinetes in engorged Boophilus microplus from a dairy herd in Venezuela, p. 148-156. In Camus, E., J. A. House & G. Uilenberg (editors). Vector-Borne Pathogens: International Trade and Tropical Animal Diseases. Ann. New York Acad. Sci. Vol. 791. New York, USA. [ Links ]
Núñez, J. L., M. Muñoz-Cobeñas & H. Moltedo. 1982. Boophilus microplus: The Common Cattle Tick. Springer-Verlag Editors. Berlin, Germany. 204 p. [ Links ]
OKelly, J. C. & G. W. Seifert. 1969. Relationships between resistance to Boophilus microplus, nutritional status, and blood composition in Shorthorn x Hereford cattle. Aust. J. Biol. Sci. 22: 1497-1506.
Roberts, J. A. 1968. Acquisition by the host of resistance to the cattle tick Boophilus microplus (Canestrini). J. Parasitol. 54: 657-662. [ Links ]
Roberts, J. A. & J. D. Kerr. 1976. Boophilus microplus: passive transfer of resistance in cattle. J. Parasitol. 62: 485-489. [ Links ]
Roush, R. T. 1993. Ocurrence, genetics and management of insecticide resistance. Parasitol. Today 9: 174-179. [ Links ]
Solomon, K. R. 1983. Acaricide resistance in ticks. Adv. Vet. Sci. Comp. Med. 27: 273-296. [ Links ]
Villares, J. B. 1941. Climatología Zootechnica. III. Contribucao ao estudo da resistencia e susceptibilidade genetica dos bovinos ao Boophilus microplus. Bol. Ind. Anim. (Sao Paulo) 4: 60-80. [ Links ]
Wharton, R. H. & K. R. Norris. 1980. Control of parasitic arthropods. Vet. Parasitol. 6: 135-164. [ Links ]
Wikel, S. K. 1996. Host immunity to ticks. Annu. Rev. Entomol. 41: 1-22. [ Links ]
1 Universidad Centroccidental "Lisandro Alvarado", Decanato de Ciencias Veterinarias, Apartado postal 665, Barquisimeto, Lara, 3001-A. Venezuela. Fax: 58 51 425989, e-mail: rmelende@delfos.ucla.edu.ve
2 Universitá de Milano, Facoltá di Medicina Veterinaria. Milan. Italy.
3 Ministerio de Agricultura y Cría (MAC). Servicio Autónomo de Sanidad Animal del Estado Lara (SASA). Barquisimeto. Venezuela.














