Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.46 n.2 San José Jun. 1998
Abstract
Aspects of the biology of Ovachlamys fulgens (Gude 1900) were studied under laboratory conditions in Costa Rica for 16 months. The lifespan of this snail in captivity was about nine months (N= 22) and it could lay viable eggs without mating (N= 40). Oviposition began 42 days after eclosion, when shell diameter averaged 5.12 mm (N= 24). Clutches had about three eggs each (N= 252). One clutch was laid daily over periods of one to four days, with intervals of one to two days (N= 252). Age, shell diameter and clutch size were positively correlated (N= 1097) as were shell diameter of adult, egg diameter and shell diameter of the offspring (N= 136). Oviposition took place between crevices of the soil and in the leaf litter. Eggs had a soft egg shell and were laid semi-hydrated; they hydrated by absorbing water from the surroundings (N= 21). This strategy may be the evolutionary compromise between a mechanical limitation (small body size) and the advantage of producing bigger eggs with a larger amount of nutritive substances. At room temperature (mean = 21°C) egg eclosion took place in 14 days (N= 67) and in 11 days at 28 °C (N= 32). Egg shell dissolution was not uniform (N= 107), it began in a local area, that usually was the side on which it rested (N= 46). The fact that eggs with and without an embryo (N= 5) underwent the same process of egg shell's calcium dissolution suggested a chemical mechanism. Embryos did not induce the process, but accelerated it. Egg viability was decreased by the tannins of the surrounding leaf litter (N= 50). Juveniles could feed on leaves of Persea americana (Lauraceae) (N= 299).
Key words
Land snail, life cycle, reproduction, egg development, oviposition, egg viability, egg shell dissolution, Helicarionidae, Ovachlamys, Costa Rica.
Information on the biology of Central American terrestrial pulmonates is scarce and limited to a few agricultural and medical papers about two species of Veronicellidae (Andrews et al. 1985, Andrews & Pilz 1987, Monge-Nájera 1996) and Succinea costaricana (Villalobos et al. 1995). Ovachlamys fulgens (Gude 1900) appears to have been introduced to Costa Rica from Asia in the last 15 years (Barrientos 1996). The spatial distribution and season population dynamics of the species in Costa Rica have been described elsewhere (Barrientos 1996). Despite its wide distribution and its world-wide agricultural importance as a quarantine pest of Heliconia spp., Dracaena marginata and orchid plantations, there are no published studies of its biology. The literature has yielded only two papers about O. fulgens, and they deal only with its taxonomy (Gude 1900).
This paper describes laboratory observations of reproductive output, parental investment and development of eggs and juveniles in O. fulgens. The effect of terrarium size on growth and reproduction, and juvenile feeding behaviour are also reported.
Materials and Methods
Specimens were collected at Pavas, San José, Costa Rica (9°56' 45"N, 84°07' 15" W, 1075 masl) in an urban orchard that has been described by Barrientos (1996). The specimens were moved to a laboratory 7 km east to the University of Costa Rica in San Pedro de Montes de Oca, San José, Costa Rica. The laboratory is located at 1200 masl and belongs to the biotic unit defined as "subtropical, tropical, humid with one to two dry months" (Herrera & Gómez 1993).
Voucher specimens were deposited in the Museum of Natural History, University of Florida and Instituto Nacional de Biodiversidad (INBio) catalogue numbers 1474249 (17 specimens in alcohol) and 1474252 (19 specimens in alcohol).
Descriptive statistics have the format: mean (sample size + standard deviation, minimum, maximum). All correlations are Spearman's.
Oviposition, viability and eclosion: Specimens isolated since the egg stage were placed in two types of terraria: standard 10 X 1 cm Petri dishes ("small terraria") and transparent white plastic boxes (10x10x6cm3, "large terraria"). Small terraria had a layer of leaf litter over another of soil (0.25 cm deep each); in large terraria these layers were 1.5 cm deep each. Densities were one individual per 39.27 cm3 and one individual per 300 cm3, respectively. Terraria were daily checked to maintain moisture. The life cycle was followed from February 1993 through January 1994. Terrarium size effect was measured over the first 60 days after eclosion. In most cases the following data were recorded every 24 hr: age, number of (a) clutches per day, (b) eggs per clutch, and diameters of (a) shell (b) eggs (c) and shell of neonates (always within the first 24 hr post-eclosion). Clutches were placed under observation in independent Petri dishes until eclosion. White transparent plastic boxes 30x21x12cm were used to keep several individuals together in an attempt to observe matings.
Details of the egg-laying behaviour were based on ten observations, two of which were video-taped to produce the illustrations. The time required for the egg to travel through the gonoduct in the "head-anterior part of the foot" section was recorded under a dissection microscope, as well as the time between oviposition of eggs in the same clutch.
Eggs were measured immediately after being laid (here called "semi-hydrated eggs") and after 2-3 min of hydration (here called "hydrated eggs") over a moist dead leaf of avocado (Persea americana) (Lauraceae) that, like the rest of plant material used in the study, was from the natural habitat. Egg volume was calculated with the formula for elliptical volume (Volume = 4/3 x (x length/2 x breadth/2 x breadth/2).
The rate of maternal investment per egg was calculated as the weight of the semi-hydrated egg divided by the weight of the mother before oviposition. The rate of maternal investment per clutch is equal to the summed weight of semi-hydrated eggs laid divided by the weight of the mother before oviposition. Only nine records of the weight of semi-hydrated eggs and 12 of parent snail before oviposition were obtained, making correction factors necessary.
The incubation period was measured under two treatments: room temperature (mean 21°C) and under constant temperature (28°C + 1o C in an incubation chamber). In the latter treatment the eggs were placed in the incubator within 24 hr after oviposition.
To check if gravity affected the dissolution of calcite crystals in the egg shell during development, 28 eggs were left in their original position and 18 were turned upside-down. All the eggs used in this experiment were 24 hr old at most and were kept at room temperature.
Viability was studied in 330 isolated eggs laid by 22 adults.
The role of substrate on egg mortality was measured by randomly distributing eggs over three substrates: I- dead mango leaves (Mangifera indica) (Anacardiaceae) (still with their original consistency and shape and colored light brown); II- dead avocado leaves (P. americana) (Lauraceae) (still with their original consistency and shape and colored light brown); and III- dead avocado leaves that had lost their original consistency and shape and had turned black. In all cases the leaves were moistened and kept in Petri dishes at 100% relative humidity.
Adult humidity preference: Standard sized Petri dishes, divided in four equal compartments by plastic divisions, were prepared with 7 ml of soil per compartment. The soil was dried for 48 hr at 90°C and sieved (all experiments with sieved soil used a 0.098 cm mesh size). Moisture in the compartments varied from dry through very humid as follows: I- 1ml water, II- 3ml, III- 4ml and IV- 5ml. Moisture categories were randomly assigned. In controls, all sections received 4ml of water. Into the middle of each Petri, an adult O. fulgens (shell diameter >5mm) was introduced. The dish was covered with a thick black cardboard to eliminated the effect of light; the position of the snail was recorded 15 min later. Specimens that stayed ambiguously between two categories were excluded form the analysis.
Juvenile feeding: Young snails used to investigate feeding were no older than 24hr; their shell diameter averaged 1.5mm (range 1.2-1.7mm). Treatments used Petri dishes with dead avocado leaves moisted to saturation. In the controls, leaves were substituted by sieved soil (taken from beneath avocado trees) also moisted to saturation. Twenty young were placed in each Petri dish and left there for five days; the intestinal content of the live animals were then examined with the dissection microscope.
Results
Oviposition, viability and eclosion: During the 16 research months there were no observations of courtship or copulation in either the field or laboratory. On the other hand, all the individuals isolated since the egg stage laid viable eggs that subsequently hatched. No external signs of sexual maturation (like reflected lip) were noticed; thus, mean shell diameter of individuals reared in the large terraria were recorded at the time of first clutch production and used as indicator of size at sexual maturity. This criterion was based on observations in the present study and on Farnesi et al. (1984) data, who showed a strong correlation between gonad maturation and body size in stylommatophores. O. fulgens laid the first eggs in clutches of one to two eggs at approximately 42-45 days after eclosion (Table 1). Terrarium size had little effect on these variables but did affect shell diameter which fell from 5.12 mm in the larger terraria to 4.73 mm in the smaller ones (Table 1).

Shell diameter and clutch size after 60 days were considerably greater for snails from the larger terraria (Table 1). Nevertheless, the number of clutches per day was independent of terrarium size (Table 1); generally, a daily clutch was produced over periods of one to four days followed by pauses of one to two days. In the smaller terraria, oviposition periods were shorter and pauses longer. Terrarium size also affected the size of young: in smaller terraria, length of hydrated eggs and shell diameter of newly emerged young were also smaller (Table 1).
Animals reared in large terraria lived, on average, 264.9 days (22, +72.53, 113-338 days) and during their lifespans produced a total of 164.8 eggs (22, +42.63, 81.0-242 eggs). They reached an average shell diameter of 6.89mm (24, +0.54, 5.2-7.5mm).
Correlations among age, adult and offspring shell diameter, and clutch, and egg size were few in the smaller terraria, with the exception of adult age, shell diameter and clutch size which increased with time (Table 2). On the contrary, in the larger large terraria most of these variables were significantly correlated (Table 2). With time, there was an increase in shell diameter but the number of clutches deposited in 24 hr was smaller, nevertheless clutch and egg size were increasingly larger (Table 2). The shell diameter of newly hatched snails was also correlated with parental age and body size, number of eggs in the clutch and diameter of the egg (Table 2).
Variables Large terraria N (r) p Small terraria N (r) p
Adult age vs. Clutch size 1097 (0.2366) ** 150 (0.1838) *
Adult age vs. Clutches per day 1097 (-0.1200) ** 150 (0.1238) NS
Adult age vs. Adult shell diameter 1097 (0.9000) ** 150 (0.5713) **
Clutches per day vs. Clutch size 1097 (-0.1180) ** 150 (-0.0806) NS
Clutch size vs. Adult shell diameter 1097 (0.3292) ** 150 (0.5590) **
Clutches per day vs. Adult shell diameter 1097 (-0.1258) ** 150 (0.1019) NS
Adult age vs. Hydrated egg diameter 561 (0.3950) ** 99 (0.1169) NS
Clutch size vs. Hydrated egg diameter 561 (0.1949) ** 99 (0.0598) NS
Clutches per day vs. Hydrated egg diameter 561 (-0.1340) ** 99 (0.0594) NS
Adult shell diameter vs. Hydrated egg diameter 561 (0.4131) ** 99 (0.2012) *
Adult age vs. Offspring shell diameter 136 (0.4367) ** 47 (0.1040) NS
Clutch size vs. Offspring shell diameter 136 (0.3519) ** 47 (0.3135) *
Clutches per day vs. Offspring shell diameter 136 (0.0370) NS 47 (-0.0511) NS
Adult shell diameter vs. Offspring shell diameter 136 (0.4058) ** 47 (0.1609) NS
Egg diameter vs. Offspring shell diameter 136 (0.5455) ** 47 (0.3001) *
N= sample size, r= correlation coefficient, p= probability. ** = p less than 0.01, *= p less than 0.05 and NS= non significant probability.
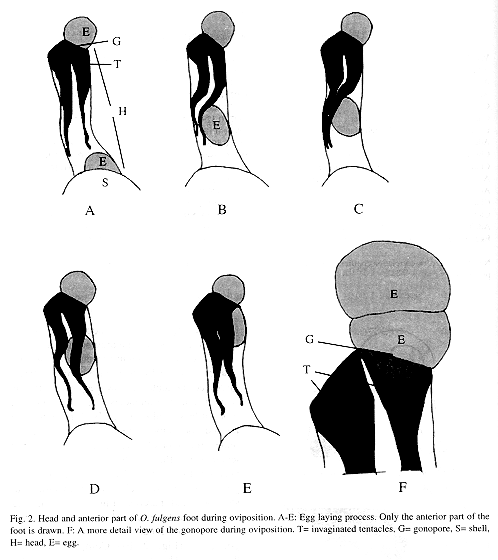
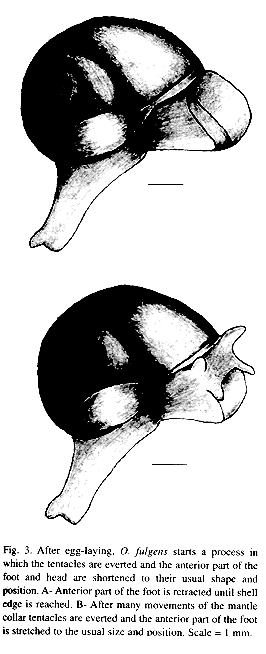
To oviposit, these snails searched out crevices in the soil or the lower sides of dead leaves (Fig. 1 A and B). Normally the caudal horn was held in position D of Fig. 5; the posterior part of the foot and shell are against the substrate while the anterior part of the food and the head were bent and placed in the selected site (Fig. 1). They could maintain this position for more than one hr (n=1) before egg-laying. The complex "head - anterior part of the foot" could reach three times the normal length, tentacles were completely invaginated and stretched in parallel position (Fig. 2 A). Eggs travelled singly via the gonoduct but usually left the uterus before the previous egg was laid (Fig. 2 A). While moving through the second quarter of its visible trip, the egg momentarily displaced the tentacles towards upper left side section, thus the tentacles were bent but kept more or less parallel (Fig. 2 B). Finally the egg pushed the right tentacle over itself, going under the tentacles (Fig. 2 C). The right tentacle was the first to recover its stretched position (Fig. 2 D-E). When the egg exited the gonopore the tentacles were invaginated, stretched and parallel (Fig. 2 E). The egg emerged in longitudinal position and was slightly squeezed while traversing the gonopore (Fig. 2 F). Complete passage through the "head - anterior part of the foot" lasted 7.31 min (6, +2.11, 4.82-10.0 min) (Fig. 2 A-F). Eggs were deposited in 5.72 min intervals (6, +3.35, 0.23-9.0 min). When the clutch had been produced the snail put its head, anterior part of the foot and tentacles in the usual position (Fig. 3 A-B) in 2.22 min (3, +0.71, 1.5-2.92 min). It began by retracting head and anterior part of the foot to the level of the shell; the dorsal part of the anterior part of the foot was contracted more rapidly giving the impression that it rolled over itself (Fig. 3 A). In this part of the process the mantle collar had many contractions followed by abundant movements of the cephalic region that ended when the tentacles came out (in no particular order) (Fig. 3 B). Finally the "head-anterior part of the foot" was stretched (specially the dorsal part) until reaching the normal position and size; the snail immediately abandoned the oviposition site.
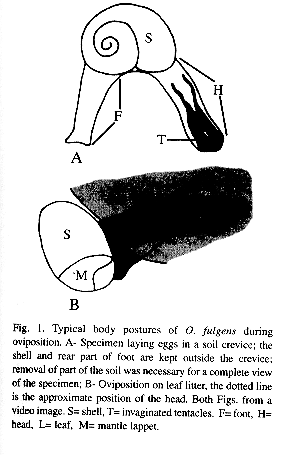
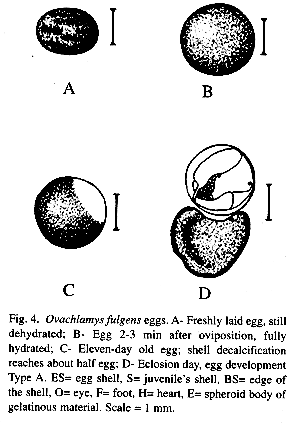
Snails were less disturbed by external activity during egg-laying; they did not react to the light of the microscope or when the terrarium was moved to improve observation, but terminated egg-laying if touched. Recently laid eggs were oval shaped and had many longitudinal folds because they were not completely hydrated (n=17) (Fig. 4 A). They had a white, flexible and thin egg shell. They hydrated fully in 2-3 min, absorbing water from their surroundings (Fig. 4 B). As a consequence, egg size increased significantly with volume exceeding 100% (Table 3). The size of the hydrated egg failed to predict size of semi-hydrated egg and viceversa (Table 4).
Variable Semi-hydrated egg ø (N, +S.D., Min-Max) Hydrated egg ø (N, +S.D., Min-Max) U-Mann-Whitney (p)
Width 1.31 mm (17, +0.099, 1.2-1.5 mm) 1.75 mm (21, +0.086, 1.5-1.9 mm) (0.0000)
Length 1.66 mm (17, +0.1, 1.4-1.8 mm) 1.87 mm (21, +0.12, 1.5-2.0 mm) (0.0000)
Volume 1.51mm3 (17, +0.273, 1.06-2.00 mm3) 3.03 mm3 (21, +0.429, 1.77-3.78 mm3) (0.0000)
ø= average, N= sample size, S.D.= standard deviation, Min= minimum, Max= maximum, p= probability.
Maternal investment per laid egg was calculated at 2.31% of its own weight (105, +0.0064, 1.2-4.4%). Investment per clutch was 10.53% (23, +0.045, 2.3-22.0%). Clutch size averaged 4.57 eggs (23, +2.52, 1-9 eggs). Heavier snails laid more eggs (Table 4). Larger clutches required greater maternal investment but investment per egg was smaller (Table 4).
Variables N (r) p
Clutch size vs. Adult weight before laying eggs 23 (0.6032) **
Clutch size vs. Adult weight after laying eggs 23 (0.6032) **
Clutch size vs. Maternal investment per clutch 23 (0.9578) **
Adult weight before laying eggs vs. Adult weight after laying eggs 12 (0.9636) **
Clutch size vs. Maternal investment per egg 105 (-0.5014) **
Adult weight before laying eggs vs. Maternal investment per egg 12 (-0.764) **
Adult weight after laying eggs vs. Maternal investment per egg 105 (-0.6828) **
Clutch size vs. Semi-hydrated egg weight 9 (0.5547) NS
Clutch size vs. Hydrated egg weight 104 (0.092) NS
Maternal investment per clutch vs. Adult weight before laying eggs (with correction factor) 23 (0.5304) *
Maternal investment per clutch vs. Adult weight after laying eggs 23 (0.4481) *
Hydrated egg width vs. Semi-hydrated egg width 17 (-0.0804) NS
Semi-hydrated egg length vs. Semi-hydrated egg width 17 (0.3454) NS
Hydrated egg length vs. Semi-hydrated egg width 17 (0.2442) NS
Hydrated egg volume vs. Semi-hydrated egg width 17 (0.0356) NS
Semi-hydrated egg length vs. Hydrated egg width 17 (-0. 3049) NS
Hydrated egg length vs. Hydrated egg width 17 (0.4544) NS
Semi-hydrated egg volume vs. Hydrated egg width 17 (-0.1610) NS
Hydrated egg length vs. Semi-hydrated egg length 17 (-0.1354) NS
Hydrated egg volume vs. Semi-hydrated egg length 17 (-0.2890) NS
Semi-hydrated egg volume vs. Hydrated egg length 17 (0.2112) NS
Hydrated egg volume vs. Semi-hydrated egg volume 17 (-0.0408) NS
N= sample size, r= correlation coefficient, p= probability, ** = p less than 0.01, *= p less than 0.05, NS= non significant probability.
In the first nine days after oviposition all eggs appeared to develop similarly. After day nine, the shell began to lose calcium from a focal point, where upon two types of egg development were recorded (n= 107). In "Type A development" the shell gradually lost calcium until approximately one half was decalcified and became transparent (the calcified section was white, Fig. 4 C). The shell broke in the translucent part and the juvenile emerged surrounded by an spheroid body of gelatinous material (Fig. 4 D), which liquefied in less than 24 hr freeing the snail. No active participation of the neonate in exiting the egg was seen. During eclosion the neonates kept their heads inside the shells with the posterior part of the foot outside; once that the gelatinous material melted, a great number of tentacle movements began and continued until they emerged and the neonate began to move away. "Type B development" began like type A but the dissolution of egg shell calcium dissolution continued until the whole shell was translucent. With a maximum of 72 hr after complete shell calcium loss the gelatinous body melted and the animal became free with the head protruding from the shell. The only exception to these types of egg development was a single embryo-less egg whose egg shell dissolution began at two points and moved until only a white equatorial band of calcium was left; five other embryo-less eggs however, had type A development and one had type B.
Egg development at constant temperature (11.34 days (32, +0.482, 11-12 days)) was significantly shorter (U-Mann-Whitney p=0.0000) than at room temperature (13.94 days (67, +0.919, 11-15 days)). Development time, temperature and eclosion type were interdependent = (Tridimensional contingency Table p=0.0000). Type A development was correlated with high temperatures (28o C) and a developmental time of about 11 days; type B was correlated with lower mean temperatures (21o C) developmental times of about 14 days. Embryo-less eggs "development" took 31.8 days (6, + 12.97, 19-48).
Egg decalcification was correlate with egg position on the substrate (x2, p=0.0238). Eggs that stayed in the original position where laid usually had the lower hemisphere translucent (n= 28, 89.28%), a trend that was less clear in those that had been turned upside down (n=18, 61.11%).
In the laboratory, egg viability was 88% (n=330 eggs). Nonviable eggs were: either infertile (2.43%), turned dull yellow or orange after absorbing tannins from the substrate (3.03%), dried out (0.61%), consumed by fungi (1.21%) or died from unknown causes (2.12%). On two occasions an infertile egg was found to contain two ova (in other words Siamese twins).
Eggs laid on dead avocado leaves, had a viability of 82.35% (n=17) when these leaves retained their consistency, shape and were pale brown in colour (treatment II); 94.12% (n=17) if such characteristics were lost and the color was black (treatment III), and 0% (n=16) on dead mango leaves (treatment I). Treatments II and III were not significantly different (x2, p=0.29) but they did differ from treatment I (x2, p=0.0000). In treatment I eggs were enveloped in a dark substance 11 days after being laid. Besides, egg color had turned from the normal white to dull greyish yellow. Saprophytic fungi developed on some eggs; others turned orange or brown. After 23 days dissection showed that no embryos had developed in any of them.
Adult humidity preference: In the control experiment snails were allowed to choose among four compartments each with equal humidity. These specimens (N= 59) showed no preference for any of the sections (Kruskal-Wallis, n=16, p=0.1490). Section I was selected by 24% of snails, II 32%, III 27% and IV 17%. By contrast, treatment specimens (n=941) showed a clear preference for sections III and IV (Kruskal-Wallis, n=40, p=0.0000) (Tukey, p<0.01). Section I had an incidence of 15%, II 23%, III 30% and IV 32%, reflecting a tendency to select the more humid parts.
Juvenile feeding: Young in petri dishes with avocado leaves tended to have full intestines (78.2%, n= 142) while most of those on soil had empty intestines (66.2%, n= 157) (x2, n=299, p= 0.0000). In the latter case specimens never had completely full intestines. By contrast those feeding on leaves showed pigmented shells and covered the walls of the trerraria with abundant faeces.
Discussion
Both in the field (Barrientos 1996) and the laboratory, reproduction was recorded during the rainy season. However, in the field snails that began to reproduce at the beginning of the rainy season were those adults that had survived the dry season. In the laboratory, reproduction was by individuals that were hatched, developed and matured during the dry season outside. Besides the physiological simplicity of adult survival (versus that of eggs or young), there is an advantage because this results in a mean of 75 more offspring and 12 300 grand-offspring assuming a 100% survival in only one year (data calculated with the means obtained in the present study).
When isolated individuals were kept and terrarium size was reduced, although the density grew the effect of interference by another individual disappeared. In O. fulgens terrarium size affected number of eggs laid, shell diameter at first reproduction, and diameter of eggs and new-born shell. In contrast with these results, Baker and Hawke (1991) found that the development of Cochlicella acuta is not affected by population density as long as food is not a limiting factor. In the present study O. fulgens always received more lettuces than what they could daily eat and leaf litter was changed weekly. As literature about helicarionids is scarce, comparison with other stylommathophorans is necessary. Baur and Baur´s (1992) work fully agrees with the results found here. They reported that several life cycle characteristics of Balea perversa are a function of population density, for example at high density sexual maturity requires more time and is reached with a smaller body size, while the number of eggs laid decreases. Studies on Theba pisana and Cernuella virgata also showed a proportional inverse relation between density and number of offspring (Baker 1991). Similar results were obtained with Limicolaria flammea which reduces the growth rate, retards sexual maturation and increases mortality when population density increases (Egonmwan 1992).
Many of the characteristics or trends that a species follows during its life cycle, such as clutch size, egg size and age at first reproduction are correlated with body size (Farnesi et al. 1984, Baker 1991, Bengtsson and Baur 1993). O. fulgens follows the known pattern because sizes of eggs, clutches and offspring shells were greater when the parents had larger shells.
Species of small terrestrial pulmonates generally have a short life cycle that often lasts one or two years (Johnson and Black 1991). Tropical species frequently have short life cycles and even some with large bodies such as Euglandina rosea, Achatina fulica and Archachatina marginata mature in one to three years (Johnson and Black 1991). Of course there are exceptions such as Rhagada convicta (Camaenidae) that requires five years for sexual maturation (Johnson and Black 1991) or Hawaiian Achatinella that are small but need four to seven years (Cowie 1992). In turn O. fulgens has a yearly life cycle, not surprising because of its size and -currently- tropical condition, but it matures sexually very rapidly in comparison with other species of similar size studied in the Neotropics (Villalobos et al. 1995).
Reciprocal insemination is the rule among pulmonates (Hyman 1967), and the exceptions include self-insemination, parthenogenesis, unilateral copulation, self mutilation of the male organs, etc. (Hyman 1967, Peake 1978, Leonard 1991). The difference between parthenogenesis and self-insemination is difficult to establish (Peake 1978), thus many papers mention "self-insemination" as a generalisation of absence of copulation, as is the case with O. fulgens. In the field, O. fulgens clutches often have little more than half the number of eggs in clutches obtained in the laboratory with specimens isolated from birth (Barrientos 1996); the greater laboratory productivity may result from the lack of predators, but this also indicates that the absence of copulation does not reduce fertility and that this could be the main, if not the only, type of reproduction in the species. Helicarionidae is not the only family with members that can reproduce without copulation; the system is also used by Punctum pygmaeum (Endodontidae) (Baur 1987), Rumina decollata (Selander et al. 1974) and several Succineidae (Bayne 1973, Villalobos et al. 1995). The Partulidae include species in which self insemination is the dominant reproductive method; for example, isolated virgin Partula gibba lay eggs at the same rate as those that have mated. Based on the low allozyme variability it has been concluded that the four species of Samoanna from the Society islands reproduce mainly by self-insemination (Cowie 1992). On the other hand, it has been shown that some species such as Helix aspersa, that normally mate (Peake 1978), can lay eggs without mating if they receive prostatic homogenates (Lucarz 1991).
Like most stylommatophores, O. fulgens oviposits in dark humid places (e.g. soil crevices, rotten trunks and leaf litter) that protect the eggs from desiccation and sharp changes of temperature (Dundee 1986, Bidart et al. 1992). O. fulgens is opportunistic because it only uses places that naturally fill the requirements of protection, not like some Helicidae that actively excavate holes for oviposition (Hyman 1967).
Stylommatophoran oviposition and maternal investment have seldom been treated in the literature (Tompa 1974a). The increase in diameter and volume of O. fulgens eggs has a different origin and must not be mistaken with the phenomenon described by Bidart et al. (1992) when the egg of Polymita muscarum increases to 14.8% its original diameter because of embrionary development. O. fulgens is outstanding because its eggs are laid semi-hydrated and reach their definitive size a few minutes later with water taken from the surroundings. Egg size at oviposition seems to be the "morphological and physiological maximum" for the species. A similar phenomenon has been reported for small turtles whose pelvic limitations force them to lay more elongated eggs (Hailey and Loumbourdis 1988). In O. fulgens evolution has favoured a strategy to increase maternal investment per egg; making an optimum use of space by including less water (but probably more nutrients) in the egg; water is not a limiting resource because egg-laying tales place during the rainy season. By having a permeable (perhaps hydroscopic) shell the egg can absorb water from the surroundings and duplicate its volume. The hydrating capacity may be because the eggs are "partially calcified" according to the categories proposed by Tompa (1974b) because CaCO3 in calcite form does not make a hard shell. As suggested for the kiwi (Apteryx australis) (Rahn et al. 1975), another possible explanation is that the ancestors of O. fulgens were larger and O. fulgens has not had the evolutionary pressure or the genetic mutation needed to decrease egg size. This last option seems less probable but its evaluation requires more studies about the egg width / adult shell diameter rate of other helicarionids to establish if the phenomenon is constant throughout the family. Also their phylogenetic affinities need to be clarify.
Another evidence for the previous analysis is obtained if we compare the proportion of egg width with shell diameter in other heliciform molluscs. Usually, as adult shell diameter decreases, the relative egg size increases (Table 5). Thus in large species such as H. aspersa egg diameter is only 1/9 of shell diameter while smaller species such as P. pygmaeum egg diameter is 1/3 of shell diameter. When O. fulgens lays its eggs these are 1/4 of shell diameter, with hydration egg diameter increases to 1/3 of shell diameter, which is the proportion known for species that have shell diameters 3.7 times smaller. Future studies should focus on the strategies that allow smaller species to produce eggs that are comparatively large.
Species (Source) Egg width (mm) Adult shell Rate: ggwidth/
diameter (mm) adult shell diameter
Helix aspersa (Dekle 1969) 3.2 28.0 1/9
Anguispira alternata (Tompa 1974a, Burch pers. com.1995) 2.8 20.0 1/7
Arianta arbustorum (Baur & Baur 1986) 2.7 18.5 1/7
Polymita muscarum muscarum (Bidart et al. 1992) 2.7 17.0 1/6
Ovachlamys fulgens Semi-hydrated egg 1.31 5.12 1/4
Ovachlamys fulgens Hydrated egg 1.75 5.12 1/3
Punctum pygmaeum (Baur 1987) 0.44 1.39 1/3
It is harder to explain why ovipositing O. fulgens fully invaginate the four tentacles while other stylommatophorans such as the endodontid Anguispira alternata (Tompa 1974a) and the helicid H. aspersa (pers. obs.) do not. An answer may require detailed anatomical studies, but it is probably related with the relatively big size of the eggs.
In agreement with this study, several researchers have found that the snails are less disturbed by the same stimuli once oviposition begins (Hyman 1967, Tompa 1974a). O. fulgens clutches tend to be small if compared with those of Limax maximus (100-368 eggs) and H. aspersa (11-96 eggs) (Hyman 1967), however, clutches are deposited with a greater frequency, similarly to the zonitid Zonitoides nitidus that lays eggs throughout the year in five to six egg clutches every 9-13 days (Didier & Rondelaud 1987). O. fulgens' strategy is adapted to habitats with a longer time suitable for egg-laying, as tropical rain forests are (Barrientos 1996).
Egg viability in O. fulgens is considerably high. Although in other species it has been found that dry climate induces individuals to lay immature eggs before aestivation (Raut & Ghose 1980), this was not observed in O. fulgens and the causes for expulsion of infertile eggs needs further study. The facility with which O. fulgens eggs absorb water from the surroundings make them be easily affected by tannins from the substrate: it would be interesting to learn how they select substrates for oviposition, if they do. Just like some species have shown feeding preferences (Speiser & Rowell-Rahier 1991) others may have oviposition preferences. On the other hand mango may have molluscicide properties like those of Anacardium occidentale, another Anacardiaceae (Laurens et al. 1987).
Tompa (1979) reported that eggs of Stenotrema leai undergo a processes of egg shell dissolution similar to O. fulgens. He suggested that some larval organ is specifically applied against the egg shell causing local calcium resorption in one area at a time. Nevertheless, the fact that embryo-less eggs undergo the same process of egg shell calcium dissolution as fertile eggs suggests that the phenomenon has a chemical nature and that embryo development does not affect it significantly. The effect of embryonic presence is only reflected in an acceleration of the process. The analysis of the egg fluid of Strophocheilus oblongus showed that the pH's fluid decreased during development (Tompa 1979), suggesting also chemical nature of the phenomenon. Tompa (1979) assumed that the pH was homogeneous in all the egg fluid, but the experiments here hint to heterogeneity due to gravity effect. However, more detail experiments are needed for a conclusion.
Many terrestrial molluscs feed on leaf litter (Szlavecz 1986, Speiser & Rowell-Rahier 1991), however, the alkaloids, tannins and phenolic compounds from the leaf are not accepted by them (Molgaard 1986, Speiser & Rowell-Rahier 1991). In this study, O. fulgens was found to accept dead avocado leaves; a valuable information for laboratory rearing of this and other leaf litter species.
Resumen
Durante 16 meses se estudió en laboratorio la biología básica de Ovachlamys fulgens en Costa Rica. Esta especie se reprodujo sin copular (N= 40) y su longevidad fue de nueve meses (N= 22). La oviposición inició a los 42 días de haber eclosionado y con 5.12 mm de diámetro de concha (N= 24). Los huevos fueron depositados en camadas diarias de alrededor de tres huevos (N= 252). Existe una correlación positiva entre la edad y el diámetro de la concha del progenitor y el tamaño de la camada (N= 1097), y entre el diámetro de los huevos y el de la concha de adultos y neonatos (N= 136). Los huevos de cáscara suave son depositados semi-deshidratados y se hidratan absorbiendo agua del entorno (N= 21). Esta estrategia puede ser el resultado evolutivo entre una limitación mecánica (pequeño tamaño corporal) y la ventaja de producir huevos más grandes con más sustancias nutritivas. Los huevos tardan 14 días en eclosionar a 21oC (N= 67). La disolución de la cáscara del huevo no es uniforme (N= 107), sino que comienza en un área específica, que normalmente es en la que reposa (N=46). El hecho de que huevos sin embrión (N= 5) pasen por el mismo proceso de disolusión de la cáscara sugiere que éste es de carácter químico. El embrión no induce el proceso, pero si lo acelera. Los taninos de la hojarasca que rodea los huevos reduce su viabilidad (N= 50).
Acknowledgements
This study was financed by the author and by Consejo Nacional para Investigaciones Científicas y Tecnológicas (CONICIT) project 94-520-BID. Instituto Nacional de Biodiversidad (INBio) provided time and facilities to finish it. I specially thank Fred G. Thompson (University of Florida) for taxonomic assistance. Carlos Villalobos (Universidad de Costa Rica -UCR-), William Bussing (UCR), familia Barrientos Corrales, Julieta Carranza (UCR) and the department of genetics of the Escuela de Biología (UCR) lent some equipment and facilities. Luis Murcia assisted with the illustrations. Julián Monge-Nájera (UCR) provided key guidance throughout the study. David Boag (Canada) and one annonymous reviewer provided excellent reviews of earlier draft. This paper is part of a M.Sc. thesis defended at the University of Costa Rica.
References
Andrews, K.L., H. Barletta & G. E. Pilz (eds.). 1985. Memoria del seminario regional de fitoprotección, abril 1984. Ceiba (Tegucigalpa, Honduras) 26: 57-112. [ Links ]
Andrews, K.L. & G.E. Pilz (eds). 1987. Memoria del II Seminario Centroamericano sobre la babosa del frijol, 22-25 Abril 1985. Ceiba (Tegucigalpa, Honduras) 28: 145-320. [ Links ]
Baker, G.H. 1991. Production of eggs and young snails by adult Theba pisana (Müller) and Cernuella virgata (da Costa) (Mollusca: Helicidae) in laboratory cultures and field populations. Aust. J. Zool. 39: 673-679. [ Links ]
Baker, G.H. & B.G. Hawke. 1991. Fecundity of Cochlicella acuta (Müller) (Mollusca: Helicidae) in laboratory cultures. Invert. Reprod. Develop. 20: 243-247. [ Links ]
Barrientos, Z. 1996. Distribución y ciclo de vida del caracol terrestre Ovachlamys fulgens (Gude, 1900) (Stylommatophora: Helicarionidae). M.Sc. Thesis, Universidad de Costa Rica, San José, Costa Rica. [ Links ]
Baur, A. & B. Baur. 1992. Responses in growth, reproduction and life span to reduced competition pressure in the land snail Balea perversa. Oikos 63: 298-304. [ Links ]
Baur, B. & A. Baur. 1986. Proximate factors influencing egg cannibalism in the land snail Arianta arbustorum (Pulmonata, Helicidae). Oecologia 70: 283-287. [ Links ]
Baur, B. 1987. The minute land snail Punctum pygmaeum (Draparnaud) can reproduce in the absence of a mate. J. Molluscan Stud. 53: 112-113. [ Links ]
Bayne, C.J. 1973. Physiology of the pulmonate reproductive tract: location of spermatozoa in isolated, self-fertilising succinid snails (with a discussion of pulmonate tract terminology). The Veliger 16: 169-175. [ Links ]
Bengtsson, J. & B. Baur. 1993. Do pioneers have r-selected traits? Life history patterns among colonising terrestrial gastropods. Oecologia 94: 17-22. [ Links ]
Bidart, L., J. Fernández Milera, M. Osorio & E. Reynaldo. 1992. Datos reproductivos de Polymita muscarum muscarum Lea (Mollusca: Pulmonata: Fruticicolidae). Reporte de investigación del Instituto de Ecología y Sistemática. Academia de Ciencias de Cuba. 8p. [ Links ]
Cowie, R.H. 1992. Evolution and extinction of Partulidae, endemic Pacific island land snails. Phil. Trans. R. Soc. Lond. B 335: 167-191. [ Links ]
Dekle, G.W. 1969. The brown garden snail (Helix aspersa Muller) (Pulmonata-Helicidae). Entomol. Circular 83: 1-2. [ Links ]
Didier, B. & D. Rondelaud. 1987. Qualitative and quantitative data on the egg-laying of a pulmonate gastropod snail, Zonitoides nitidus Mueller, in order to perfect a breeding method. Acta Oecol. Appl. 8: 343-354. [ Links ]
Dundee, D. 1986. Notes on the habits and anatomy of the introduced land snails, Rumina and Lamellaxis (Subulinidae). Nautilus 100: 32-37. [ Links ]
Egonmwan, R.I. 1992. The effects of population density on sexual maturation in Limicolaria flammea Mueller (Gastropoda: Pulmonata: Achatinidae). J. Afr. Zool. 106: 297-301. [ Links ]
Farnesi, R.M., S. Tei & F. Panella. 1984. Relation between gametogenesis and size of individuals in Helix aspersa. Ann. Fac. Agrar. Univ. Stud. Perugia 38: 41-52. [ Links ]
Gude, G.K. 1900. Further notes on helicoid land shells from Japan, the Loo-Choo, and Bonin Islands, with descriptions of seven new species. Proc. Malacol. Soc. 4: 70-80. [ Links ]
Hailey, A. & N. S. Loumbourdis. 1988. Egg size and shape, clutch dynamics, and reproductive effort in European tortoises. Can. J. Zool. 66: 1527-1536. [ Links ]
Herrera S., W. & L. D. Gómez P. 1993. Mapa de Unidades Bióticas de Costa Rica. Incafo S.A. et al. San José, Costa Rica. 1: 685 000. [ Links ]
Hyman, L.H. 1967. The Invertebrates Vol. 6: Mollusca I. McGraw-Hill, New York. 791p. [ Links ]
Johnson, M.S. & R. Black. 1991. Growth, survivorship, and population size in the land snail Rhagada convicta Cox, 1870 (Pulmonata: Camaenidae) from a semiarid environment in Western Australia. J. Moll. Stud. 57: 367-374. [ Links ]
Laurens, A., J. Belot & C. Delorme. 1987. Molluscicidal activity of Anacardium occidentale L. (Anacardiaceae). Ann. Pharm. Fr. 45: 471-473. [ Links ]
Leonard, J.L. 1991. Sexual conflict and the mating systems of simultaneously hermaphroditic gastropods. Am. Malacol. Bull. 90: 45-58. [ Links ]
Lucarz, A. 1991. Evidence of an egg-laying factor in the prostatic secretions of Helix aspersa Mueller. Comp. Biochem. Physiol. Comp. Physiol. 100: 839-844. [ Links ]
Molgaard, P. 1986. Food plant preferences by slugs and snails: a simple method to evaluate the relative palatability of the food plants. Biochem. Syst. Ecol. 14: 113-122. [ Links ]
Monge-Nájera, J. 1996. Molluscs of economic and sanitary importance in the Tropics: the Costa Rican experience. Universidad de Costa Rica, San José. 166p. [ Links ]
Peake, J. 1978. Distribution and Ecology of the Stylommatophora. p. 429-526. In: V. Fretter & J. Peake (eds.). Pulmonates Vol. 2 A Systematics, Evolution and Ecology. Academic Press, London. [ Links ]
Rahn, H., C.G. Paganelli & A.Ar. 1975. Relation of avian egg weight to body weight. Auk 92 (4): 750-765. [ Links ]
Raut, S.K. & K.C. Ghose. 1980. Influence of unfavourable environment on oviposition in some gastropods. Indian J. Anim. Sci. 50: 1121-1123. [ Links ]
Selander, R. K., D.W. Kaufman & R. S. Ralin. 1974. Self-fertilisation in the terrestrial snail Rumina decollata.. Veliger 16: 265-270. [ Links ]
Speiser, B. & M. Rowell-Rahier. 1991. Effects of food availability, nutritional value, and alkaloids on food choice in the generalist herbivore Arianta arbustorum (Gastropoda: Helicidae). Oikos 62: 306-318. [ Links ]
Szlavecz, K. 1986. Food selection and nocturnal behavior of the land snail Monadenia hillebrandi mariposa (Pulmonata: Helminthoglyptidae). Veliger 29: 183-190. [ Links ]
Taylor, R.J. 1991. Distribution and habitat of Helicarion rubicundus (Pulmonata: Helicarionidae), a rare land snail. Pap. Proc. Roy. Soc. Tasmania 125: 27-28. [ Links ]
Tompa, A. 1974 a. Oviposition in the land snail Anguispira alternata (Stylommatophora: Endodontidae). Malacol. Rev. 7: 35-36. [ Links ]
Tompa, A. 1974 b. The structure of calcareous snail eggs. Malacol. Rev. 7: 49-50. [ Links ]
Tompa, A. 1979. Localized egg shell dissolution during development in Stenotrema leai (Pulmonata: Polygyridae). Nautilus 94 (4): 136-137. [ Links ]
Villalobos M., C., J. Monge-Nájera, Z. Barrientos & J. Franco. 1995. Life cycle and field abundance of the snail Succinea costaricana (Stylommatophora: Succineidae), a tropical pest. Rev. Biol. Trop. 43: 181-188.
1 Departamento de Malacología, Instituto Nacional de Biodiversidad, Apdo. 22-3100 Sto. Domingo, Heredia, Costa Rica. Fax: (506) 244 25 48. Zbarr@spiraxis.inbio.ac.cr














