Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.46 n.1 San José Mar. 1998
Resumen
Se cultivó plántulas de Qualea grandiflora a partir de semillas de "Cerrado", con distintas cantidades de agua, en invernadero o cámara de crecimiento, eligiendo 5 o 10 plantas al azar. Reaccionaron al limitado aporte hídrico disminuyendo la conductividad estomática y los potenciales hídrico y de turgencia. En las plantas bajo deficit hídrico no se observó ajuste osmótico. La relación raiz: vástago fue más alta bajo estrés hídrico, presentando la parte aérea menor crecimiento debido a la menor producción y expansión foliares.
Key words
Cerrado, Qualea grandiflora, water stress, abscisic acid.
Qualea grandiflora is a typical tree from the Brazilian cerrados. The cerrado vegetation is subjected to prolonged and often severe drought lasting up to four months (Ferri 1961). Although cerrado tree species do not show water stress at the beginning of the dry season they suffer prolonged drought stress (Ferri 1955, Peres and Moraes 1991). This delayed response reflects a very deep root system (Ferri 1961), an adaptation of species subject to low water availability (Pereira and Pallardi 1989). More recent data has shown that in the dry season species from the cerrado reduce stomatal conductance (Perez and Moraes, 1991; Franco 1995) and photosynthetic rate (Franco 1995).
This paper describes Qualea grandiflora's reaction to low water availability with regard to parameters such as vegetative growth and levels of abscisic acid, a plant hormone that seems to be associated with water stress alterations (Munns and Sharp 1993).
Plant material: Seeds of Qualea grandiflora Mart. were collected from ten trees located in a cerrado area at Itirapina, São Paulo state, Brazil, and stored at room temperature until required. Before sowing, seeds were treated for 5 min with concentrated sulfuric acid and washed in running tap water for 24 hr. The naked seeds were then germinated in Petri dishes. Seven-day-old seeds were transferred to narrow polyethylene tubes with sand or cerrado soil from the Itirapina region. The tubes were 22 cm long and 3.7 cm in diameter, and were perforated at the base. Batches of eight to twelve tubes were held vertically in glass jar supports.
Greenhouse experiments: In water stress experiments carried out in a greenhouse, the control jar contained distilled water. In the water deficiency treatment, the tubes were wetted to field capacity and then 5cm3 of water were added to each tube every ten days. The experiments lasted for eighty-five days, and were carried out at Campinas, São Paulo state, 22°54'S; 12.0 - 13.5hr photoperiod.
Growth-room experiments: In the water stress experiments carried out in controlled environment cabinets (University of Edinburgh, Scotland) the seedlings were grown in river-washed quartz sand and were supplied daily with 1/5 strength solution based on that of Hoagland and Arnon (1938) adjusted to pH 4.8-5.0. In the water deficiency treatment, on the first day of the experiments, after setting up, the tubes were weighed and were only wetted again when there was a loss of 10 g of water; this meant a 40% water deficiency in relation to the control, since the control presented 25 g of water. The experiments lasted for sixty-five days. The temperature was 30/26°C (day/night) and the photoperiod 12 hr. Photon flux density from warm white fluorescent and tungsten lamps was 260 mol.m-2.s-1.
Growth analyses and water relations: The mean relative growth rate (R), the mean unit leaf rate (E) and the mean leaf area ratio (LAR), were calculated for ten plants according to Hunt (1982). In the analyses tops did not include cotyledons: these were measured separately.
Water potential, osmotic pressure and turgor pressure were measured in five plants growing in growth-rooms, according to Milligan and Dale (1988). Transpiration and stomatal conductance were determined in the leaves of five plants with a steady state porometer. Two measurements were made per plant.
Abscisic acid determination: Cotyledons (five) of known weight from different plants (grown in growth-rooms) were frozen in liquid nitrogen and macerated in plastic tubes with a glass rod; bi-distilled water (2 cm3 per 200 mg) was added, and the tubes were kept at 4°C under darkness for 12 hr with constant stirring. The samples were centrifuged at 200 g for 10 min and the supernatant was used for ABA immunoassay determination, according to Quarrie et al. (1988).
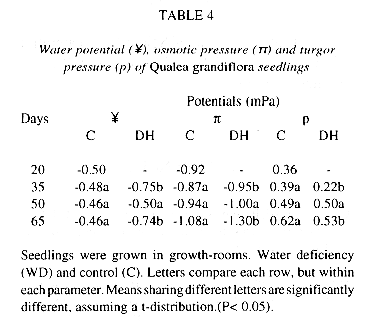
Qualea grandiflora, growing under low addition of water, presented low biomass (Table 1) and a sharp decrease in unit leaf rate (Table 2). This appears to be an adaptation to a low water supply, consisting of a reduction of the stomatal conductance, and consequently a reduction in transpiration (Table 3). The consequence is a higher leaf water potential that diminishes the possibility of injury caused at the cellular level, normally a consequence of dehydration (Levitt 1980). However, at the same time that the reduction of the stomatal conductance restricts the loss of leaf water, this mechanism also restricts the entrance of CO2, leading to a decrease in photosynthetic rate and thus of growth (Chapin et al. 1987, Nautyal et al. 1994), as observed in Q. grandiflora. In our experiments, the species also showed a decrease in leaf expansion (Table 1) under water stress, possibly associated with the decrease shown in turgor potential (Table 4), because of the effect of the cell turgor pressure upon cellular expansion (van Volkenburgh and Cleland 1984). The growth of tops, both because of decreased leaf expansion and slower leaf emergence, was more affected by water deficiency than root growth. This resulted in a higher root/shoot ratio (Table 1).
| | | |||
| | | | | |
| Dry weight(mg) | ||||
| Root | | | | |
| Shoot | | | | |
| Cotyledons | | | | |
| Leaves | | | | |
| Seedling | | | | |
| Area (cm2) | ||||
| Cotyledons | | | | |
| Leaves | | | | |
| Total | | | | |
| Root/Shoot | | | | |
Greenhouse (gh), growth-rooms (gr), water deficiency (WD) and control (C). Letters compare each row; small letters only compare gh; capital letters, only gr. Means sharing different letters are significantly different, assuming a t-distribution. (P< 0.05).
| | | |||
| | | | | |
| R (mg.md-1.d-1) | ||||
| Root | | | | |
| Shoot | | | | |
| Cotyledons | | | | |
| Seedling | | | | |
| E (mg.cm-2.d-1) | | | | |
| LAR (cm2.mg-1) | | | | |
Greenhouse (gh), growth-rooms (gr), water deficiency (WD) and control (C). Letters compare each row; small letters only compare gh; capital letters, only gr. Means sharing different letters are significantly different, assuming a t-distribution. (P< 0.05).
| | Conductance (mmol.m-2s-1) | |||
| | | | | |
| | | | | |
| | | | | |
| | | | | |
Seedlings were grown in growth-rooms. Water deficiency (WD) and control (C). Letters compare each row, but within each parameter. Means sharing different letters are significantly different, assuming a t-distribution. (P< 0.05).
Another cerrado tree species growing under low water supply, Dalbergia miscolobium, has also shown higher root/shoot ratios (Sassaki 1995). However, this behavior seems not to be widespread for cerrado tree species because Bauhinia rufa and Serjania caracasana, also cerrado trees do not show a higher root/shoot ratio under water stress (Sato and Moraes 1992). It has been suggested that abscisic acid is involved in the control of the root/shoot ratio, mainly when the plant is under water stress (Smith and Dale 1988, Creelman et al. 1990, Munns and Sharp 1993). Unfortunately in our case, the presence of unknown interfering substances in Q. grandiflora complicated the ABA analyses and resulted in high values of standard error.
From the findings of this work, and also because of previous findings related to the anatomy and morphology of Qualea grandiflora, as the stomata are localized in hair crypts on the inferior leaf epidermis (Morretes and Ferri 1959) and very deep root systems (Ferri 1961), it can be said that this species presents adaptations to retard water loss inside tissues when a water deficiency occurs, while drought tolerance adaptations, such as osmo-regulation (Pereira and Pallardi 1989) were not found in Qualea grandiflora.
Acknowledgements
We thank the International Scientific Cooperation EC-Brazil (ISC CII-0620) and CAPES for financial support.
References
Chapin, F.S. III, Bloom, A.J., Field, CB. & Waring R.H. 1987. Plant responses to multiple environmental factors. Bioscience 37: 49: 57. [ Links ]
Ferri, M.G. 1955. Ecology in "cerrado and caatinga". Comparative study about water balance in these vegetation. Bol. Fac. Filos. Cienc. Let. USP, Bot. 12: 1-70. [ Links ]
Ferri, M.G. 1961. Aspects of the soil-water-plant relationship in connection with some Brazilian types of vegetation, p. 103-109. In: Tropical soils and vegetation Proceedings of the Abidjian Symposium ( UNESCO, ed.). Abidjian. [ Links ]
Franco, A.C. 1995. Fotossíntese em plantas de cerrado, p. 385.In: Resumos do XLVI Congresso Nacional de Botânica ( SBB, ed.). Brasil. [ Links ]
Hoagland, D.R. & Arnon, D.I. 1938. The water-culture method for growing plants without soil. Univ. Calif. Agric. Expt. Stn. Circ. 347. [ Links ]
Hunt, R. 1982. Plant growth curves: the functional approach to plant growth analysis. Edward Arnold, London. 248p [ Links ]
Levitt, J. 1980. Responses of plants to environmental stresses.Vol. II. Academic, London. [ Links ]
Milligan, S.P. & Dale, J.E. 1988. The effects of root treatments of the primary leaves of Phaseolus vulgaris L. Biological analysis. New Phytol. 109: 35-40. [ Links ]
Morretes, B.L. & Ferri, M.G. 1959. Contribuição ao estudo da anatomia de folhas de plantas de cerrado. Bol. Fac. Fil. Ciênc. Letr. USP, Bot. 16: 7-70. [ Links ]
Munns, R. & Sharp, R.E. 1993. Involvement of abscisic acid in controlling plant growth in soil of low water potential. Aust. J. Plant Physiol. 20: 425-437. [ Links ]
Nautiyal, S, Badola, H.K., Pal, M. & Negi, D.S. 1994. Plant responses to water stress: changes in growth dry matter production, stomatal frequency and leaf anatomy. Biologia Plantarum 36: 91-97. [ Links ]
Pereira, J.S. & Pallardi, S. 1989. Water stress limitation to tree productivity. In: Biomass production by fast growing trees (J.S. Pereira & J.J. Landsberg eds.). Kluwer Academic, London, p. 37-56. [ Links ]
Perez, S.C.J.G.A. & Moraes, J.A.P.V. 1991. Curso diário e sazonal do potencial de água e da condutância estomática em espécies de cerradão. Rev. Brasil. Biol. 51: 805-811. [ Links ]
Quarrie, S.A., Whitford, P.N., Appleford, N.E.J., Wang, T.L. Cook, S.K., Henton, I.E. & Loveys, B.R. 1988. A monoclonal antibody to (s)-abscisic acid: its characterisation and use in a immunoassay for measuring abscisic acid in crude extracts of cereal and lupin leaves. Planta 173: 303-339. [ Links ]
Sato, A. & Moraes, J.A.P.V. 1992. O efeito do estresse hídrico sobre as trocas de CO2 gasoso em plantas jovens de espécies do cerrado. Arq. Biol. Tecnol. 35: 763-775. [ Links ]
Smith, P.G. & Dale, J.E. 1988. The effects of root cooling and excision treatments on the growth of primary leaves of Phaseolus vulgaris l. New Phytol. 110: 293-300. [ Links ]
Van Volkenburgh, E. & Cleland, R.E. 1984. Control of leaf growth by changes in wall properties. What's New in Plant Physiology 15: 25-28. [ Links ]
1Universidade Federal de Santa Catarina, Depto de Botânica, 88040-900, Florianópolis, SC, Brazil.
2 Instituto de Botânica, C.P. 4005, 01061-970, São Paulo, SP, Brazil.
3 University of Edinburgh, Division of Biological Sciences, Edinburgh, Scotland.














