Hemophilia A is a recessive X-linked bleeding disorder caused by decreased levels of coagulation factor VIII. It is responsible for spontaneous and prolonged bleeding, especially in patients with the severe form of the disease where factor VIII levels are less than 1%.1 To stop or prevent bleeding, factor replacement therapy is used, and one of the most significant complications of this treatment is the production of alloantibodies against FVIII which are called inhibitors and have an impact on approximately 20-30% of patients.1,2 In the presence of inhibitors, the infusion of FVIII becomes less effective because the activity of FVIII is neutralized. In order to stop acute bleeding agents that bypass the need for FVIII, such as activated prothrombin complex concentrate (aPCC) or activated recombinant factor VII (rFVIIa) are used. Bypassing agents are more expensive than regular treatment, for example a vial of 500 U of FVIII (regular treatment) costs $100, a patient might need 2 vials three times per week. On the other hand, a vial of aPCC (500 U) costs $800 and rFVIIa (2mg) costs $1000. A patient might need up to 4 vials of aPCC every 12 hours or 90ug/kg of rFVIIa every 2 hours while they are bleeding.1 To eliminate such inhibitors immunetolerance induction, known as ITI, is the only treatment shown to be successful in up to 80% of cases.3 ITI consists of the regular prolonged exposure of the patient to clotting factor concentrate to induce tolerance and to eradicate the inhibitors.1
Although several ITI regimens have been proposed, consensus has not been reached on which dose and type of factor concentrate should be used during ITI. According to the international immunetolerance study, there was no difference in the success rates between the high dose (200 IU/Kg/day) and the low dose (< 50 IU/kg/thrice weekly) regimens of FVIII, in children or adults with high response inhibitors.4,5
Case presentation
A 10-year-old boy with neurofibromatosis type 1 and severe hemophilia A developed FVIII inhibitors at age 3 in 2010. He was previously treated with on demand FVIII therapy at the national hemophilia treatment center, where high response inhibitors were documented. He was then transferred to our hospital in 2013 after a bleeding during a dental extraction, due to social problems. In that occasion he required a blood transfusion and high doses of FVIII. Even though our patient was very active he used to bleed no more than 2 or 3 times per year. In our center low response inhibitors were detected in 2014, but it was not until September 2015 when ITI could be started. Inhibitor titer at the initiation of ITI therapy was negative and the level of FVIII was 1.75%.
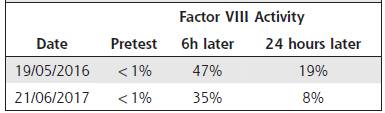
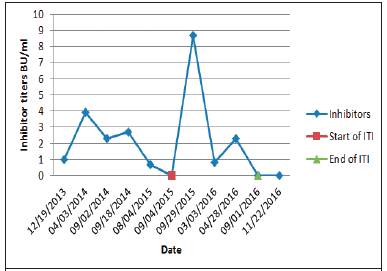
A low dose FVIII regimen with 750 IU/day (50 IU/kg) Monday - Wednesday - Friday was initiated on September 3rd, 2015. No aPCC or rFVIIa were used as prophylactic treatment during ITI. aPCC was only used in case of acute bleeding. Six months after ITI was initiated, inhibitor titers were 0.8 BU/mL and they continued to decrease progressively. The recovery test in the 8th month of ITI was in the normal range, showing that the inhibitor became negative (see table 1). ITI was continued until September 2016 when the FVIII inhibitor titer was undetectable. FVIII inhibitor titers evolution before, during, and after ITI therapy are illustrated in Figure 1.
Nowadays our patient is on FVIII prophylaxis, and he is event free (no bleeding).
Discussion
Prophylactic replacement therapy is the gold standard of care in patients with severe hemophilia, it not only prevents hemorrhage but also its related arthropathy.5,6 The development of FVIII inhibitors in patients with hemophilia A is a very difficult and expensive complication of their treatment.2,4,7 Even though the presence of an inhibitor does not modify the frequency or severity of the hemorrhage, it could increase morbidity and mortality and make their management even more challenging.3
Once an inhibitor develops, regular prophylaxis with FVIII needs to be discontinued and only by-passing agents can be used, which rise both on demand and prophylaxis costs and also represent a less effective haemostatic therapy.2,8ITI is the best way to eradicate the inhibitors permanently, to restore prophylactic FVIII therapy, to stop bleedings and thus to improve the patient’s quality of life.2,7,8
Several good prognostic factors for ITI have been described, as: 1) FVIII inhibitor titer at the beginning of therapy less than 10 BU/ml; 2) historical peak inhibitor titer less than 200 BU/ ml; 3) peak inhibitor titer during ITI less than 250 BU/ml; 4)
Initiation of ITI within 5 years of FVIII inhibitor appearance. The success of ITI is also considered better in younger patients with early ITI.2,3,5
Our patient almost met all of the criteria of good prognostic FVIII inhibitor titer at the beginning of ITI was undetectable even though we do not know the titers in the other center. According to our data, 3.9 BU/mL was the highest titer before ITI, during ITI the highest titer was far less than 250 BU/mL and ITI was started five years after FVIII inhibitors were detected.
It is known that less bleeding has been reported in patients using a high dose regimen and FVIII tolerance has been achieved faster as well.3 However, a high dose FVIII regimen is very difficult to afford for a country like ours, and we cannot expect that a patient pays for his own treatment either, so our patient underwent ITI with a low dose FVIII regimen. A month after ITI was initiated, inhibitor titer was 8.7 BU/ml; the highest titer ever registered in our center showing a normal anamnestic response which is considered to be a common immunological reaction associated with ITI. There are regimens using ITI therapy with high dose FVIII combined with intravenous immunoglobulin. For this regimen a positive elimination of the anamnestic response (less exaggerated) has been described. 5
ITI should always be continued for at least 9 months and no more than 33-36 months according to different authors.2,3,7 In our patient the recovery of FVIII in plasma was normalized within eight months after the initiation of ITI; nevertheless, therapy was continued until September 2016 in order to complete a full year of therapy and by that time FVIII inhibitors were measured again, turning back negative.
ITI complete success is achieved when a negative inhibitor test (<0.6 BU/ml), FVIII recovery is >66%, half life ≥ 6 hours measured after a 72h treatment free washout period and no anamnestic reaction for the next FVIII application is observed. A partial response is achieved with a negative inhibitor test and FVIII recovery <66% or half life ≤6h enabling FVIII use over a period of 6 months of on demand treatment or 12 months of prophylaxis after ITI termination. Failure to fulfill the criteria for full or partial response within at least 36 months of ITI treatment is considered treatment failure.2,3,7Our patient met criteria for a partial response; he had a negative inhibitor titer with a FVIII recovery test less than 66%.
In a developing country, on demand treatment is more likely to be given: it is enough to stop acute bleeding but poorly prevents arthropathy. A primary prophylaxis regimen early in life should be the number one treatment option for hemophilic patients, and ITI without delay should always be considered once inhibitors were developed. Even though it is an expensive therapy, its cost is compensated by the chronic expensive care a patient with FVIII inhibitors would represent.5,7,9
In a country like Costa Rica, with limited economical resources, this patient represents a complete success, and he demonstrates that patients with good prognostic factors might be effectively treated with low-dose FVIII regimen.
Conflict of interest: The author reports no conflict of interest.