Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Acta Médica Costarricense
On-line version ISSN 0001-6002Print version ISSN 0001-6012
Acta méd. costarric vol.60 n.1 San José Jan./Mar. 2018
Articles
Wilms tumor in Costa Rican children
1Hospital Nacional de Niños "Dr. Carlos Sáenz Herrera". Caja Costarricense de Seguro Social Costa Rica
Wilms tumor (WT) is the most common kidney tumor in the pediatric age, representing 7% of all tumors in this age group.1-3 An incidence of 7.1 cases per million in children under 15 years of age was reported in the United States, a group in which the diagnosis is more frequent.1 The predominant clinical finding is the abdominal mass, reported in up to 90% of cases, which may be unilateral and painless.4-6 Bilateral tumors were also reported in up to 5% of children, and other more rare, such as metachronous tumors, in a lesser percentage.1,6,7
There are some syndromes associated with the appearance of WT. These include the Beckwith-Wiedemann syndrome, Denys-Drash syndrome and the syndrome that includes Wilms tumor, aniridia, genitourinary malformations and mental retardation (WAGR). Other less reported conditions (1%) are usually family cases where there is a history of tumors in first or second degree relatives.2,3 Beyond this, the appearance of the WT has been related to the WT1 and WT2 genes.
In Costa Rica, there is an experience of more than 20 years in the management of WT, however, to date there are no studies that analyze the clinical data of patients who have had it, or the evolution or survival of these children to the treatment used.
It is important to mention that the chemotherapy protocol used in Costa Rica is different from the European protocol and that used in the rest of Central America. Therefore, what is provided by the study will be very valuable for the population, since the findings can be analyzed in order to determine if it is necessary to change the management of the patients.
In the present work an analysis of the clinical and epidemiological characteristics of the patients diagnosed with the WT was carried out from January 1991 to December 2011. The response to the treatment used was analyzed, also the events presented and the overall survival was determined and free of disease at 5 years.
Methods
The following is a descriptive, retrospective study with a 20-year cohort based on the review of the files, in any of its formats (digital, physical or microfilmed). The population of children ages 0 to 13 years old diagnosed with WT who had undergone a confirmatory biopsy from January 1991 to December 2011 was included. Files with incomplete information or to which access was not obtained were excluded, as well as the patients that refused treatment.
The study was approved by CLOBI-HNN-006-2014. The list of cases was obtained from the Statistics Department of the same Center. It was based on a sample of 129 reported cases, however, after the exclusion process, only 69 cases were included in the study.
The following information was obtained from all the files: identification number, sex, age, clinical presentation at diagnosis, date of diagnosis (established according to the date of the biopsy), stage, histology, first or second line chemotherapy, presence of risk factors (intraoperative tumor rupture, presence of microscopic debris, positive adenopathies, tumor volume greater than 800 cc), use of radiotherapy, sites of metastasis, events (first and second), failure and date of last event or date of failure.
To obtain information on the different risk factors analyzed, not only the histopathological reports were reviewed, but also the operative notes to corroborate the presence or absence of tumor rupture.
Two groups were established for the analysis of chemotherapy. Group A for patients with early stages (I to III) and group B for patients with stages IV, V and unresectable tumors. In addition, depending on the stage at diagnosis and the size of the tumor mass, patients could receive pre-surgical chemotherapy. During this, Vincristine 1.5mg/m2 and Actinomycin 1.5mg/m2 were administered. After completing it, the patient was taken to the operating room, where a partial or complete nephrectomy was performed. Patients who did not receive presurgical chemotherapy were operated immediately and then started with first-line chemotherapy. In the study patients, adjuvant chemotherapy was dependent on stage, histology, mass size and the presence of metastases. Adjuvant chemotherapy was based on Vincristine 1.5mg/ m2, Actinomycin 1.5mg/m2 and Epirubicin 50mg/m2. Both pre-surgical and adjuvant chemotherapy are part of a Costa Rican protocol designed and implemented as of 1995. The National Wilms Tumor Study Group (NWTSG) scale was used to define the stage.
Complete response was defined as the absence of relapse events, progression or persistence of the disease; partial response, when a second-line of chemotherapy was required, but without further complications, and failure, for patients who died or who were in a palliative condition of their disease.
Overall survival was defined from the date of diagnosis until the date of the last control appointment, and eventfree survival, from the date of diagnosis until the last event or failure. Both were calculated at 5 years.
The descriptive statistical techniques used for the analysis of the information are the frequency distribution; crossing of variables between sex, age, stage, and therapeutic protocol used. On the other hand, to determine the degree of association between the variables, the Spearman’s correlation coefficient was used. In order to perform the inferential statistics we used the comparison of means based on the analysis of variance, and previously the Levene test was used to establish the homogeneity of variances. To predict the probability of failure, a logistic regression was calculated. The statistical processing of the data was carried out in SPSS version 17.0 and in Excel. For the survival analysis, the Kaplan Meier curves were used.
Results
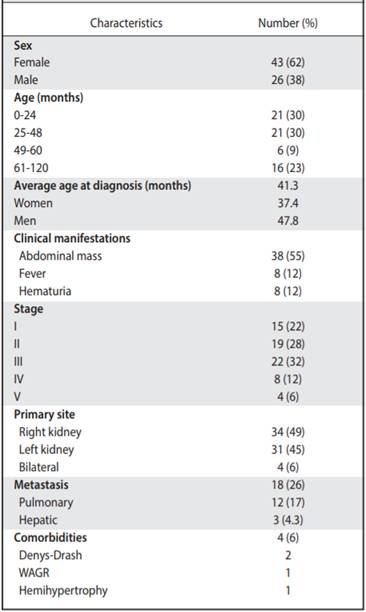
Clinical and epidemiological characteristics Of the 69 cases analyzed, 43 women (62%) and 26 men (38%) were found. The age range at diagnosis was 0-8 years old (average 41.3 months or 3.4 years, Table 3) The average age of diagnosis for women was 37.4 months and for men, 47.8 months.
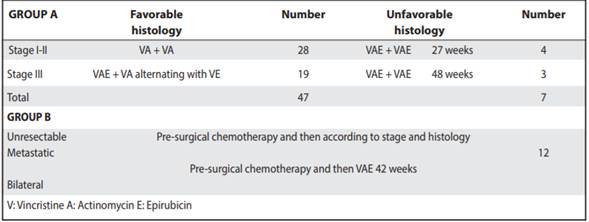
Table 1 Chemotherapy protocol for patients with Wilms tumor treated from 1991 to 2011. Oncology. Service-NCH
The majority of the population started with an abdominal mass (55%), followed by hematuria (12%) and fever (12%). Only one of the patients presented hypertension. Two cases were found where patients presented abdominal pain after trauma and two others where acute appendicitis was initially suspected. On three occasions, the tumor was found incidentally as part of studies of preauricular appendages and rectal polyps (4%, Table 2).
The most common type of tumor was stage III (31.8%), with stages I to III (81%) predominating in the study. It was found that in 5.7% of the cases, stage V, which corresponds to bilateral tumors (Table 2). In addition, the histology that was mostly reported was favorable (80%). With respect to the site of a primary tumor, in 34 patients (49%) the right side was affected and in 31 (45%), the left side. The most practiced surgical procedure was total nephrectomy (97%), mostly without intraoperative complications. Only 9% of tumors with a tumor volume greater than 800 cc were documented. And in their relationship, luckily there was no tumor rupture in 67% of cases.
Within the analysis of risk factors, there was evidence that in few cases positive adenopathies (13%) or microscopic residues (23%) were described in the histopathological reports.
Comorbidities were associated in 6% of the patients. Two patients with Denys-Drash syndrome, one with hemihypertrophy and one with the syndrome that includes Wilms tumor, aniridia, genitourinary malformations and mental retardation.
Treatment, evolution and survival
As part of the treatment used, pre-surgical chemotherapy was used in 51% of the children. The most used adjuvant chemotherapy protocols correspond to those of early stages and favorable histology (Table 1). Radiation therapy was not used in most patients.
There was a complete response to chemotherapy in 69.6% of patients. The partial response was obtained in 8.7% of the children, and 20% presented treatment failure. Only one of the patients was lost during follow up (Table 3).
A first event was documented in 20 cases; 15 attended with relapses and 5 with persistence of the disease. Of this group, 5 patients died after their first event. Subsequently, 8 presented a second event, of which 3 relapses were manifested, 2 with progression of the disease, one with persistence of disease and 2 were considered in palliative condition. Unfortunately, all died (Table 3). In total, 14 patients died and one refused treatment.
It was found that 18 patients presented metastases, being at the pulmonary level the most frequent (17%), followed by liver metastasis (4.3%, Table 2). Some complications after chemotherapy were described in 6% of patients (intestinal occlusion, renal failure and heart failure and scoliosis). None of the patients associated second malignancies and only in one case there was renal failure and congestive heart failure (Table 3). The overall survival of the patients was 73.3%, with an average follow-up of 7.4 years since the diagnosis (Figure 1), and the event-free survival was 69% (Figure 2).
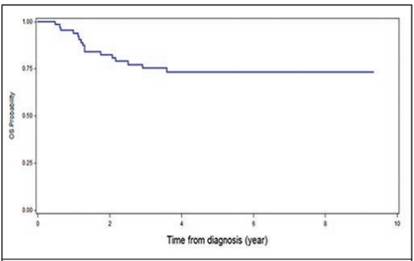
Figure 1 Overall survival (OS) at 5 years in patients with Wilms T. treated in the Oncology Service of the NCH, from January 1991 to December 2011
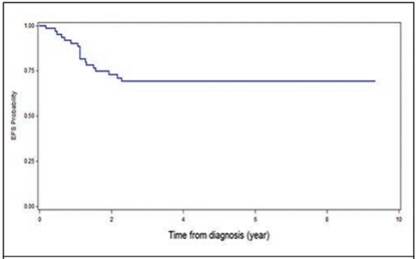
Figure 2 Event-free survival (EFS) at 5 years in patients with Wilms T. treated at the Oncology Service of the NCH, from January 1991 to December 2011
Table 2 Characteristics of patients with Wilms tumor from 1991 to 2011. Oncology Department, National Children’s Hospital “Dr. Carlos Sáenz Herrera “
Discussion
In the study, 69 files selected from the total sample of 129 patients diagnosed with WT were analyzed over a period of 20 years.
It was observed that there were more cases of women than men; however, no significant difference was demonstrated. The average age of diagnosis is 3.4 years, which is similar to that reported in the reviewed literature and in European studies.1,2,7-8 Regarding the diagnostic age according to sex, it has been described that in men they usually manifest at younger ages, however, in our environment they presented late compared to women.
Most of the patients in the study first presented an abdominal mass, a finding similar to that reported in the literature.1,6-8
Four patients in total associated comorbidities, which have been described in the literature related to the appearance of this kidney tumor.
As a finding similar to that of other studies, stage III was the most commonly identified.1,6,7 However, in our environment it was also evidenced that the early stages (I-III) are usually the most frequent. It is important to mention that 5.7% of the patients presented stage V, which corresponds to bilateral tumors, percentage in line with that reported worldwide.1, 8-9
Pre-surgical chemotherapy is used in Europe and is recommended by the International Society of Pediatric Oncology (ISPO), with the purpose of reducing the mass significantly to simplify surgery, reduce the risk of tumor rupture and therefore reduce the possibility of abdominal relapse and improve the prognosis. In its favor it has been reported that patients receiving preoperative chemotherapy require less treatment with radiotherapy and is used as a prognostic indicator of initial response to treatment. A disadvantage in administering it is the risk of underestimating the tumor stage, since it produces necrosis; however, it has been observed that it does not modify the presence of anaplasia, so it does not alter the stratification of the tumor. In addition, patients who have received chemotherapy without previous histological study, have a risk described lower than 2%, to associate complications.11-14 In cases where surgery is performed at diagnosis, the methodology used in North America, mainly, has the advantage of incorporating histopathological and molecular biology studies to define the tumor prognosis.10,11
The medications used for adjuvant chemotherapy were Vincristine, Actinomycin D and Doxorubicin. Since 1980, Doxorubicin has been used with few studies that mention its true usefulness in patients with WT and its effect on overall survival or event-free survival.
Recently, Pritchard Jones et al. analyzed in a group of patients with WT, the effect of use or disuse of Doxorubicin in the evolution of children (EFS 2 years, 5 years and OS).15 With this study it was found that overall survival is not affected when comparing patients who received Doxorubicin versus those who did not. However, more relapse was observed in patients whose chemotherapy regimen was restricted to Doxorubicin.
With their results they concluded that there was no difference in the overall survival of the children, but highrisk patients (intraoperative tumor rupture or metastatic disease) should always receive adjuvant treatment with the three drugs.
The cardiological or hepatotoxic effects of the use of Doxorubicin could not be compared in these patients, since it is described that they could appear up to 20 years after the suspension of anthracycline treatment.15 In our case, pre-surgical chemotherapy without Doxorubicin has been used in all patients. As is known, radiotherapy is part of the WT treatment. It is used in advanced stages or in conditions where there are associated risk factors. Unfortunately, the information about this therapy was not obtained, due to the fact that the patients received the treatment in another medical center and therefore, the data was in files outside the institution. It is worth mentioning that until recently, patients were managed by adult radiotherapists with an unfounded therapeutic strategy for pediatrics. However, this was recently modified.
The presence of metastasis can be found in some patients at the time of diagnosis, and in other cases, during the evolution of the disease. The study showed that 26% of patients presented metastasis, mostly in the lung, as other authors also observed in their studies.5,6
The percentage of failure of this study is comparatively greater than that reported in others, however, with a much lower percentage of patients lost during follow-up, which could be considered an advantage, since it allows to objectify the evolution of children. When comparing these results of overall survival or event-free survival with other studies, both were lower than those reported in developed countries; however, they are much greater than those described for underdeveloped countries where the overall survival is very low.5-6
When analyzing the causes of death, all the exposed variables should be considered together. When the failure with the unfavorable histology was analyzed, it was statistically significant (p = 0.042). Other risk factors analyzed with regards to patient failure were: presence of positive lymph nodes (p = 0.014), persistent microscopic debris (p = 0.04) and tumor volume greater than 800cc (p = 0.01), which resulted statistically significant too. Finally, the presence of metastasis in the patients also resulted in poor prognosis, with a statistically significant value (p = 0.000).
Finally, all this data allowed us to objectively analyze the treatment that was applied in Costa Rican patients with WT, with good initial results, for being a developing country, we have a overall survival that is closer to that of developed countries. It is relevant to ask if there is an advantage in applying protocols established by entities such as AHOPCA and ISPO, which report a little more survival than ours.
The results obtained reveal the clinical characteristics of Costa Rican children and their similarity with international reports.
Thanks to the analysis carried out with this study in the NCH, pre-surgical chemotherapy was implemented in all patients and Doxorubicin was added as part of the adjuvant therapy, so patients will be followed up in the coming years to evaluate and control the impact of these new strategies, as well as their effectiveness.
Gratitude: to Gloria Tridello, for her collaboration with the analysis of survival.
Referencias
1. Al-Hussain T, Ali A, Ahktar M. Wilms Tumor: an update. Adv Anat Pathol 2014;21:166-173 [ Links ]
2. Breslow N. Olshan A. Beckwith B. Green D. Epidemiology of Wilms tumor. Med Pediatr Oncol 1993;21:172-181 [ Links ]
3. Varan A. Wilms tumor in children: an overview. Nephron Clin Pract 2008;108: 83-90 [ Links ]
4. Fischbach BV. Trout Kl. Lewis J. Luis CA. Ski M. WAGR syndrome: a clinical of 54 cases. Pediatrics. 2005; 116,984-8 [ Links ]
5. E. Ko Michael, Ritchey M. Current management of Wilms tumor in children. J Ped Uro. 2009; 56-65 [ Links ]
6. Siegel MJ, Chung EM. Wilms tumor and other pediatric renal masses. Magn Reson Imaging Clin N Am 16 2008; 479-497 [ Links ]
7. Erginel B, Vural S, Akin M, Karadag C, Sever N, Abdullah et al. Wilms Tumor: A 24 year retrospective study from a single center. J Pediatr Hematol Onco, 2014; 31:409-414 [ Links ]
8. Ching Ching C, Ka Fan T, Hui Keung Y, Kwok Shing Chiang A, Cheung Ling S, Chau Ho Fi, et al. A 20 year prospective study of Wilms tumor and other kidney tumors: a report from Hong Kong. J Pediatr Hematol Oncol 2014; 36:445-450 [ Links ]
9. Meléndez Mambié M, Guibelalde del Castillo M, Nieto del Rincón N, Jimenez R, Femenia R, Piñana R. Tumor de Wilms bilateral metacrónico. An Esp Pediatr. 2002; 241-250. [ Links ]
10. Vujanic G. Renal tumor of childhood: an overview. Diagn Histopathol 2009:15:11 501-509 [ Links ]
11. Keebsabai S. Jurairut T. Achar S. Long-Term outcome in pediatric renal tumor survivors: experience of a single center. J Pediatr Hematol Oncol 2013: 35, 610-613. [ Links ]
12. Bhatnagar S. Management of Wilms tumor: NWTS vs SIOP. J Indian Assoc Pediatr Surg 2009; 6-14 [ Links ]
13. Lemerle J, Voute P.A, Tournade F, Rodary C, Delemarre J.F.M., Sarrazin D, et al. Effectiveness of Preoperative Chemotherapy in Wilms´ Tumor: Results of an International Society of Pediatric Oncology: Clinical Trial. JCO. 1983; 604-607. [ Links ]
14. Metzger M. Dome J. Current Therapy of Wilms Tumor. Oncologist 2005;10:815-826. [ Links ]
15. Pritchard Jones K, Bergeron C, Camargo B, Heuvel-Eibrink M, Cha T, Godzinki J, et al. Omission of doxorubicin from the treatment of stage II-III intermediate-risk Wilms Tumor (SIOP WT-2001): an open label, non inferiority, randomised controlled trial. Lancet 2015; 386: 1156-64. [ Links ]
Received: June 21, 2016; Accepted: October 26, 2017











 text in
text in