Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Acta Médica Costarricense
On-line version ISSN 0001-6002Print version ISSN 0001-6012
Acta méd. costarric vol.59 n.1 San José Jan./Mar. 2017
Articles
Serious infections in patients with rheumatoid arthritis treated with tumor necrosis factor alpha antagonist therapy
1Servicio de Reumatología, Hospital “Dr. Rafael Ángel Calderón Guardia”.
Rheumatoid arthritis (RA) is the most common autoimmune inflammatory arthropathy worldwide, affecting approximately 1% of the population.1 In Latin America, the estimated prevalence is close to 0.5%,2 Similar to that observed in Spain in 2001.3 In Brazil for 2013 it is estimated that it affected about 1% of the population.4 Unfortunately, there are no epidemiological studies in our country.
From the pathophysiological point of view, new pathways are often found involved in the immunologic cascade that characterizes RA. The role of some inflammatory mediators such as antibodies, growth factors, adhesion factors, cytokines such as tumor necrosis factor alpha (TNFα) and matrix metalloproteinase (MMP) have been defined.
TNFα plays a central role in the pathogenesis of RA.5,7 It is produced mainly by synovial macrophages and to a lesser extent by lymphocytes in response to proinflammatory stimuli;1,8,9 it’s expressed as a transmembrane protein activated by a specific MMP (TNF converting enzyme), after which it is converted into a soluble protein that is oligomerized to form a homotrimer constituting the active form. The effects of TNFα are mediated by two structurally distinct receptors: receptor 1 (TNF R-I) and receptor 2 (TNF R-II).8,10,11
The binding of TNFα to the receptor activates several intracellular signaling pathways, including the activation of transcription factors such as the nuclear factor kappa-light-chain-enhancer of activated B-cells (NFκB), protein kinases such as MAP kinase and proteases such as caspases among others.9 In RA, TNFα induces the production of proinflammatory cytokines such as interleukin 1 (IL-1), interleukin 6 (IL-6), chemokines such as interleukin 8, and increases vascular permeability and expression of endothelial adhesion molecules among other effects.12
Given its chronic condition, RA requires continued use of various medications. The current therapeutic arsenal is made up of non-steroidal anti-inflammatory drugs, traditional disease modifying anti-rheumatic drugs (DMARDs), steroids, and in recent years biological disease-modifying antirheumatic drugs or simply biological have been introduced which include TNFα antagonists (antiTNFα), therapy anit-CD20 and anti-IL-6 therapy among others,1,13 fundamental in the treatment of RA in patients who do not respond clinically to treatment with traditional DMARDs.1,7,14
AntiTNFα are drugs that have been shown in multiple controlled clinical studies to be very effective in the treatment of inflammatory diseases such as RA, juvenile idiopathic arthritis, Crohn’s disease and psoriatic arthritis.5,8,15-19 In RA, they have been shown to delay the progression of joint damage,6,20,21 they improve symptoms and signs, as well as functional status and quality of life.6,22
The therapeutic effects of antiTNFα in RA are mainly grouped into two major mechanisms: downregulates the local and systemic production of inflammatory cytokines; and decreases the activation and migration of lymphocytes at the joint level.23,24 This implies a decrease in the levels of IL-1, IL-6, MMP and in the expression of endothelial adhesion molecules.25,26 Previous studies have shown a decrease in the synovial macrophage population by induction of apoptosis mediated by the antiTNFα.8
In Costa Rica, the use of antiTNFα drugs started in 2005. Currently our social security system has three antiTNFα: Etanercept, Adalimumab and Infliximab for the management of various pathologies in specialties such as Rheumatology, Dermatology and Gastroenterology among others. In Rheumatology the antiTNFα are used for diseases including RA, Spondyloarthropathies and Adult Still’s Disease among others.
From its clinical use it became apparent that the inhibition of TNFα is associated with an increase in the incidence of severe infectious processes.4,8,27,28 In 2001 the US Food and Drug Administration of the United States (FDA) issued a warning regarding infectious processes associated with antiTNFα.18 Subsequent studies showed an increased risk for severe infections by intracellular microorganisms mainly in the upper respiratory tract, lung and skin; due to pathogens such as tuberculosis (TB) and other mycobacteria, viruses, endemic fungi and some bacteria,5,18,19,27,29-32 Because TNFα plays a greater role in the defense against microorganisms (especially intracellular) preventing spread, by activating the formation of granulomas.8,13,16,30,32,33 Some of the mechanisms described are related to activation and differentiation of macrophages, as well as the stimulation to the formation of phagosomes.34
Studies for several years have shown a higher incidence of severe infectious diseases requiring hospitalization in patients with RA versus patients without RA, causing significant morbidity and mortality.5,7,15,27,35,36 These are defined as those requiring hospitalization for management or for antibiotic therapy, which are potentially fatal and in addition, those that caused death.27,37,38 In general, severe infectious processes are considered to be related to immunological alterations of the disease, especially cellular immunity due to a decrease in the number and function of suppressor T lymphocytes and natural killer lymphocytes (NK lymphocytes),36 to the drugs used for its control or a combination of both.5,13,27,30,34,37
Some factors associated with an increased risk of severe infectious processes with antiTNFα therapy in RA patients are: steroid dose, first six months of anti-TNF treatment, recent previous surgery, lymphopenia and comorbidities such as Diabetes Mellitus (DM) and Chronic Obstructive Pulmonary Disease (COPD).4,16,18,27,29,38
Costa Rica lacks clinical studies on patients treated with antiTNFα drugs, including the development of infectious and non-infectious complications due to its prescription. The purpose of the study was to characterize the demographics of the population using antiTNFα therapy from the “Dr. Rafael Angel Calderón Guardia” Hospital, as well as to determine the clinical behavior of the infectious diseases associated with antiTNFα, in order to contribute relevant information about this population and the associated infectious diseases in our country.
Methods
The present observational and retrospective study was performed at the “Dr. Rafael Ángel Calderón Guardia” Hospital of the Costa Rican Social Security, in Costa Rica. Previous authorization from the local bioethics committee (CLOBI-37-09-2014), the database of patients using antiTNFα therapy from the local pharmacotherapy committee of the “Dr. Rafael Ángel Calderón Guardia” Hospital was analyzed and it was compared with the list that in semiannual form was sent of the service of Rheumatology to said committee. From the list the population of patients with RA treated with antiTNFα drugs was identified and treated for at least one year, during the period between 2006 and 2012; the patients were excluded that had a diagnosis other than RA, as well as those with RA treated with antiTNFα intermittently, either because of a pregnancy, therapeutic failure or for a period of less than one year. The demographic characteristics of the population was described, as well as the clinical and epidemiological characteristics of the severe infections developed after the onset of antiTNFα and the risk factors associated with severe infection(s).
The clinical and microbiological data of the severe and non-severe infectious diseases were identified in the patients that fulfilled the inclusion criteria, but only those related to severe infectious diseases were analyzed. It was defined as a severe infection in the cohort, such as that characterized by requiring hospitalization for its management or prescription of antimicrobial therapy, potentially fatal or causing death.
Based on this information, the clinical records were reviewed and the following variables were studied: gender; current age in years; age at the time of diagnosis of RA in years; age at the time of initiation of antiTNFα in years; number of antiTNFα drugs used; current antiTNFα therapy, use of DMARDs or immunosuppressants at the start of antiTNFα therapy; positivity of rheumatoid factor; interval between initiation of antiTNFα therapy and the development of severe infection in months; location of severe infection; isolated microorganism(s); antimicrobial therapy(s) used; dose of antimicrobial(s) used; duration of antimicrobial therapy prescribed in days; comorbidities associated with initiation of antiTNFα therapy including: DM, lymphopenia less than 1500 / mm3, recent surgery in the last 12 months and COPD; patients with doses of oral steroids less than 10 mg/day at the start of antiTNFα therapy; patients with doses of oral steroids greater than 10mg/day at the start of antiTNFα therapy; presence of latent TB. The required data were collected from the patients’ clinical records using a “Data Collection Sheet” prepared for this purpose and approved by the Local Bioethics Committee.
For the analysis and interpretation of the obtained data, measures were used such as average, median, frequency among others. The SPSS program for Windows 14.0 was used as a statistical analysis tool.
Results
As of June 2014, a total of 117 people had received antiTNFα therapy for at least one year, of which only 51 patients were diagnosed with RA. We found 50 files that met the inclusion criteria, 45 corresponded to women and 5 to men. The average age of the 2014 cohort was 52.9 years (SD: ± 10.9); the minimum corresponds to 35 years and the maximum 78. The average age at the time of diagnosis of RA in the population was 38.6 years (SD: ± 10.9) and at the beginning of antiTNFα therapy, 47.9 years (SD: ± 10.6).
Regarding therapy at the time of analysis, 78% of the patients used etanercept and 22% adalimumab. The use of DMARDs or immunosuppressants at the start of antiTNFα therapy averaged 1.44, with methotrexate being the most prescribed. Other parameters related to the treatment are described in Table 1.
Table 1 Characteristics of treatment in patients with RA in biological therapy in the CGH from 2006 to 2012
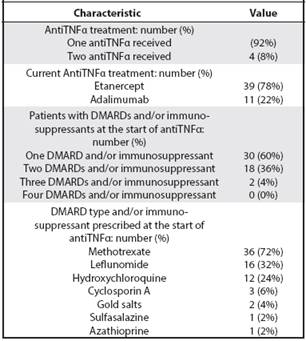
The determination of the rheumatoid factor was found in 49 clinical files, of which 37 were seropositive (75%); the remaining percentage was seronegative.
In the population analyzed, a severe infection was documented, corresponding to a skin and soft tissue infection at the right thigh level. The episode occurred 8 months after the start of antiTNFα; no microbial isolation was achieved in the various cultures performed including blood culture and secretion culture. An adequate clinical response was obtained following intravenous antibiotic treatment with oxacillin at doses of 2 grams every 6 hours and Clindamycin at doses of 600 mg every 8 hours for a period of 7 days, reaching hospital discharge of the patient.
In the analyzed population, risk factors for the development of severe infections were found: 2 patients with DM, 4 patients with a history of recent surgery and 1 patient with lymphopenia at the start of antiTNFα therapy.
Regarding the prescription of steroids at the start of antiTNFα therapy, 8 patients had no prescription. Of the 42 patients on steroids, 24 had doses lower than 10 mg/day and 15 with oral doses equal or greater than 10 mg/day. In 3 cases, parenteral steroids were used (methylprednisolone was used at 250mg daily) for clinical control of the disease, for a period of 3 days. The mean oral steroid dose in the cohort was 6.92 mg/day.
Studies of latent TB were positive in 5 patients of the analyzed population. In all of the cases they received chemoprophylaxis from the Infectology department of the “Dr. Rafael Ángel Calderón Guardia” Hospital prior to the start of antiTNFα therapy.
Discussion
The demographic profile of the cohort analyzed is similar to other international studies, with a predominantly midlife female population. Some classic examples are the CORRONA (Consortium of Rheumatology Researches of North America), ReACT (Research in Active Rheumatoid Arthritis trial) and TEMPO (Trial of etanercept and methotrexate with radiographic Patient Outcomes) studies.39-42
The period between the onset of RA and the prescription of antiTNFα therapy in the population analyzed is similar to other cohorts with an average of about 10 years.39-42 In our country, antiTNFα have been prescribed since 2005, so that patients initially treated with this therapy, correspond to those with a long-standing disease with no initial clinical response to DMARDs prior to that year. The prescription of antiTNFα therapy is limited to patients with a persistent active disease based on the DAS28 (Disease Activity Index of 28 joints), CDAI (Clinical Disease Activity Index) or SDAI (Simple Disease Activity Index) scores, which do not respond to different choices of DMARDs, recommended in national and international institutional treatment guidelines43, so that, to date the antiTNFα do not represent an option for patients with early RA in our social security system.
Similar to other biological records such as the french RATIO (Research Axed on Tolerance of Biotherapies), most patients only received one antiTNFα, in 4 cases it was necessary to change the initial therapy, either by primary or secondary failure. The antiTNFα drugs have shown through multiple studies their effectiveness in the clinical control of RA refractory to the various DMARDs, similar to the one described in this cohort.8,16,20,21 Unlike the french RATIO registry where Etanercept was the least prescribed antiTNFα, in our analysis it corresponds to the most prescribed therapy, which obeys reasons of an institutional nature.
Regarding the use of DMARDs or immunosuppressants, most patients used only one DMARD (especially methotrexate and less leflunomide) at the start of antiTNFα therapy, similarly to other records such as CORRONA.39 Predominant monotherapy is a striking feature, since it is common in the country to prescribe various combination therapies, including Odell (methotrexate, hydroxychloroquine and sulfazalazine), or combinations of methotrexate with leflunomide among others for the management of patients with persistent active disease, prior to considering the use of biological therapy. The predominant monotherapy in the cohort ,could translate an inability of the various combination regimens of DMARD to achieve prolonged clinical remission or minimal clinical activity of RA, or in turn represent intolerance to the various side effects of these. Such an observation may require further investigation in order to determine possible causes. On the other hand, the use of immunosuppressants in the analyzed population, not considered as current therapeutic options in the various international guidelines for RA, such as gold salts and Cyclosporin A (some associated with adverse effects such as proteinuria, severe cytopenias or arterial hypertension),43 because these therapies they were included in the institutional guidelines in previous years, before considering the use of biological therapy.
The immunological profile of patients is characterized by the positivity of rheumatoid factor in the majority. Currently the seropositivity is considered one of the factors that confer a worse prognosis for RA, as well as extra-articular involvement, presence of radiological erosions or frank functional limitation and the degree of clinical activity based on DAS, DAS28, among others; in which case an early aggressive therapy should be considered.43
The clinical profile of the only severe infection documented consistent with the first reports of severe infectious processes related to antiTNFα, the analysis of the German record of biological RABBIT (Rheumatoid Arthritis: Beobachtung der Biologika Therapie) in 2005 and the English record BSRBR (British Society for Rheumatology Biologics Register) in 2006 significant increases were demonstrated for skin and soft tissue infections related to antiTNFα therapy.4,5,7
Severe infection developed within 8 months of initiating antiTNFα therapy, which differs from that described in the literature. The maximum risk has been described in the first 6 months of treatment initiation.4,44 In 2007 Askling and colleagues showed that the risk of infection requiring hospitalization in patients exposed to drug antiTNFα decreases over time, 1.43 (95% confidence interval: 1.18 to 1.73) in the first year to 0.82 (95% confidence interval: 0.62 to 1.08) in the third.18
Based on the clinical response to the established intravenous antibiotic therapy, it is likely that the cause was a bacterial agent, despite not achieving microbial isolation in the cultures performed. In 2013 an analysis of the BSRBR English registry of skin and soft tissue infections in users of antiTNFα showed gram positive cocci as the main causative agents followed by pseudomonas species. Of 130 severe skin and soft tissue infectious processes, gram positive cocci were isolated in 103 patients, of which 84 corresponded to Staphylococcus aureus, 11 to Streptococcus sp. and 8 cases to negative coagulase Staphylococcus.35
The presence of initiation of antiTNFα therapy of comorbidities such as DM, COPD, as well as lymphopenia and recent surgery have been associated with an increased risk of severe infectious events.4,15,16,27-29,34,38 In the present cohort, the prevalence of comorbidities is unusually low compared to studies from other latitudes. Those made in the United States, France and England have prevalences of DM in ranges varying from 6.0% to 18.9%, and COPD between 8.0% and 28.0%.16,17,29,33 This lower prevalence of comorbidities does not have a clear explanation and could be a cause of the low incidence of severe infections, which is why later analyzes are required to corroborate or to discard such observation.
The use of steroids has been identified as a major risk factor in antiTNFα users,4,15,16 The percentage of patients receiving steroids (84%) is similar to that of studies such as RATIO, but higher than that reported in the US SABER (Safety Assessment of Biologic Therapy) registry, 60.1%. However, there is a significant difference in relation to high doses of steroids, whereas in the RATIO study up to 50% received oral doses greater than 10mg/day or intravenous boluses and in the SABER study 10.2%, our cohort in 36% received considerable high oral and parenteral steroidal doses.17
Several studies have demonstrated the importance of TNFα in TB control. In a retrospective cohort study of 112300 Canadian patients with RA, the overall TB rate was 2.2 cases per 1000 people/year (95% confidence interval 2.0 to 2.4). The incidence among patients with antiTNFα therapy was 2.6 per 1,000 people/year (95% confidence interval 1.9 to 3.3).30 In the present study, 5 patients were identified by studies with positive latent TB prior to initiation of antiTNFα therapy. The figure is similar to other Latin American reports, a small Colombian cohort showed a high prevalence of latent TB, higher than that reported in cohorts from other latitudes.45,46
From this investigation it is concluded that the demographic profile of the population using antiTNFα therapy is similar to that described in international studies, as well as the clinical and epidemiological profile of severe infections. The low incidence of severe infections could be related to a lower prevalence of comorbidities in the analyzed cohort, however future prospective national clinical-epidemiological studies are necessary to corroborate or rule out such observation.
The study presents some limitations including a small population to be analyzed, compared to classic RA studies including thousands of patients.
Other epidemiological variables in the analysis were not taken into consideration that could have enriched the results and the discussion, including the presence of sequelae secondary to RA, presence of anemic syndrome or thrombocytosis secondary to the chronic uncontrolled inflammatory process, elevation of the acute phase reactants, decrease in DAS28 after the prescription of antiTNFα therapy, among others, so it is considered a significant weakness not to have obtained a broader epidemiological characterization.
Nor was the primary or secondary failure to antiTNFα therapy adequately characterized, which might have enriched the analysis, in attempting to establish possible causes. Because of the large difference between the number of patients treated with various antiTNFα therapies, it is impossible to make comparisons.
An important point that limited a broader analysis is the absence of measurements in the clinical file such as the ACR20, ACR50 and ACR70 responses routinely used internationally in clinical trials both to assess the clinical response to antiTNFα therapy as well as therapies with DMARDs.
Work performed in: the Rheumatology Department of the “Dr. Rafael Ángel Calderón Guardia” Hospital.
Affiliation of the authors:
1Rheumatology Department, “Dr. Rafael Ángel Calderón Guardia” Hospital.
2Rheumatology Department, Mexico Hospital.
Abbreviations: RA, rheumatoid arthritis; antiTNF, tumor necrosis factor alpha antagonist; HLA, human leukocyte antigen; TNF, tumor necrosis factor alpha; MMP, Matrix metalloproteinases; DMARDs, Disease modifying antirheumatic drugs; TB, tuberculosis; DM, diabetes mellitus; COPD, chronic obstructive pulmonary diseasemarsbcr@gmail.com
Referencias
1. Seymour HE, Worsley A, Smith JM, Thomas SHL. Anti TN Fagents for rheumatoid arthritis. Br J Clin Pharmacol 2001; 51:201-208 [ Links ]
2. Delgado-Vega AM, Martin J, Granados J, Anaya JM. Epidemiología genética de la artritis reumatoide ¿Qué esperar para América Latina? Biomédica 2006; 26:562-84 [ Links ]
3. Carmona L, Villaverde V, Hernández-García C, Ballina J, Gabriel R, Lafon A, et al. The prevalence of rheumatoid arthritis in the general population of Spain. Rheumatology 2002; 41:88-95 [ Links ]
4. Goh L, Jewell T, Laversuch T, Samantha A. A systematic review of the influence of anti-TNFon infection rates in patients with rheumatoid arthritis. Rev Bras Reumatol 2013; 53:501-515. [ Links ]
5. Dixon WG, Watson K, Lunt M, Hyrich KL, Silman AJ, Symmons DPM. Rates of serious infections, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumour necrosis factor therapy. Arthritis Rheum 2006; 54:2368-2376 [ Links ]
6. Haroon N, Inman R. Infectious complications of biological therapy. Curr Opin Rheumatol 2009; 21:397-403 [ Links ]
7. Listing J, Strangfeld A, Kary S, Rau R, Hinueber U, Stoyanova-Scholz M, et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum 2005; 52:3403-3412 [ Links ]
8. Ellerin T, Rubin RH, Weinblatt ME. Infections and anti-tumour necrosis factor α therapy. Arthritis Rheum 2013; 48:3013-3022 [ Links ]
9. Zuo JX, Braun J, Sieper J. Immunological basis for the use of TNF -blocking agents in ankylosing spondylitis and immunological changes during treatment. Clin Exp Rheumatol 2002; 20:S34-S37 [ Links ]
10. Sacca R, Cuff C, Lesslauer W, Ruddle NH. Differential activities of secreted lymphotoxin-alpha3 and membrane lymphotoxin-alpha1beta2 in lymphotoxin-induced inflammation: critical role of TNF receptor 1 signaling, J Immunol 1998; 160:485-491 [ Links ]
11. Bazzoni F, Beutler B: The tumor necrosis factor ligand and receptor families, N Engl J Med 1996; 334:1717-1725 [ Links ]
12. Feldman M, Elliot MJ, Woody JN, Maini RN. Anti-tumor necrosis factor-a therapy of rheumatoid arthritis. Adv Immunol 1997; 64:283-350 [ Links ]
13. Martin-Mola E, Balsa A. Infectious complications of biologic agents. Rheum Dis Clin N Am 2009; 35:183-199 [ Links ]
14. Kavanaugh A, Cohen S, Cush JJ. The evolving use of tumor necrosis factor inhibitors in rheumatoid arthritis. J Rheumatol 2004; 31:1881-1884 [ Links ]
15. van Dartel SA, Fransen J, Kievit W, Dutmer EA, Brus HLM, Houtman NM et al. Predictors for the 5-year risk of serious infections in patients with rheumatoid arthritis treated with anti-tumour necrosis factor therapy: a cohort study in the Dutch rheumatoid Arthritis Monitoring (DREAM) registry. Rheumatology 2012; 52:1052-1057 [ Links ]
16. Salmon-Ceron D, Tubach F, Lortholary O, Chosidow O, Bretagne S, Nicolas N, et al. Drug-specific of non-tuberculosis opportunistic infections in patients receiving anti-TNF therapy reported to the 3-year prospective French RATIO registry. Ann Rheum Dis 2011; 70:616-623 [ Links ]
17. Baddley JW, Winthrop KL, Chen L, Liu L, Grijalva CG, Delzell E, et al. Non-viral opportunistic infections in new users of tumour necrosis factor inhibitor therapy: results of the Safety Assesment of Biologic Therapy (SABER) Study. Ann Rheum Dis 2013; 0:1-7 [ Links ]
18. Askling J, Fored CM, Brandt L, Baecklund E, Bertilsson L, Feltelius L, et al. Time-dependent increase in risk of hospitalisation with infection among swedish RA patients treated with TNF antagonists. Ann Rheum Dis 2007; 66:1339-1344 [ Links ]
19. Wallis RS. Biologics and infections: lessons from tumor necrosis factor blocking agents. Infect Dis Clin N Am 2011; 25:895-910 [ Links ]
20. St. Claire EW, Wagner CL, Fasanmade AA, Wang B, Schaible T, Kavanaugh A et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2002; 46:1451-1459 [ Links ]
21. Lipsky PE, van der Heijde D, St. Clair EW, Furst DE, Breedveld FC, Kalden, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Engl J Med 2000; 343:1594-1602 [ Links ]
22. Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann RM, Bulpitt KJ et al. Etanercept therapy in rheumatoid arthritis. Ann Intern Med. 1999; 130:478-486 [ Links ]
23. den Broeder AA, Joosten LAB, Saxne T, Heinegard D, Fenner H, Miltenburg AMM et al: Long term anti-tumour necrosis factor α monotherapy in rheumatoid arthritis: effect on radiological course and prognostic value of markers of cartilage turnover and endothelial activation, Ann Rheum Dis 2002; 61:311-318 [ Links ]
24. Lorenz HM, Antoni C, Valerius T, Repp R, Grunke M, Schwerdtner N, et al: In vivo blockade of TNF-α by intravenous infusion of a chimeric monoclonal TNF-α antibody in patients with rheumatoid arthritis: short term cellular and molecular effects, J Immunol 156:1646-1653, 1996 [ Links ]
25. Charles P, Elliot MJ, Davis D, Potter A, Kalden JR, Antoni C et al. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-α therapy in rheumatoid arthritis. J Inmunnol 1999; 163:1521-1528 [ Links ]
26. Catrina AI, Lampa J, Ernestam S, af Klint E, Bratt J, Klareskog L et al. Anti tumour necrosis factor (TNF)-α therapy (etanercept) down-regulates serum matrix metalloproteinase (MMP)-3 and MMP-1 in rheumatoid arthritis. Rheumatology 2002; 41:484-489 [ Links ]
27. Salliot C, Gossec L, Ruyssen-Witrand A, Luc M, Duclos M, Guignard M, et al. Infections during tumour necrosis factor α blocker therapy for rheumatic disease in daily practice: a systematic retrospective study of 709 patients. Rheumatology 2007; 46:327-334 [ Links ]
28. Bernatsky S, Habel Y, Rahme E. Observational studies of infections in rheumatoid arthritis: a metaanalisys of tumour necrosis factor antagonist. J Rheumatol 2010; 37:928-931 [ Links ]
29. Galloway JB, Hyrich KL, Mercer LK, Dixon WG, Ustianowski AP, Helbert M, et al. Risk of septic arthritis in patients with rheumatoid arthritis and the effect of anti- TNF therapy: Results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2011; 70:1810-1814 [ Links ]
30. Patkar NM, Teng GG, Curtis JR, Saag KG. Association of infections and tuberculosis with antitumour necrosis factor alpha therapy. Curr Opin Rheumatol 2008; 20:320-326 [ Links ]
31. Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006; 295:2275-2285 [ Links ]
32. Stenger S. Inmunological control of tuberculosis: rol of tumour necrosis factor and more. Ann Rheum Dis 2005; 64:iv24-iv28 [ Links ]
33. Winthrop KL, Baxter R, Liu L, Varley CD, Curtis JR, Baddley JW, et al. Mycobacterial disease and antitumour necrosis factor therapy in USA. Ann Rheum Dis 2013; 72:37-42 [ Links ]
34. Ali T, Kaitha S, Mahmood S, Ftesi A, Stone J, Bronze MS. Clinical use of anti-TNF therapy and increased risk of infections. Drug, Healthcare and Patient Safety 2013; 5:79-99 [ Links ]
35. Galloway JB, Mercer LK, Moseley A, Dixon WG, Uistianoswski AP, Helbert M, et al. Risk of skin and soft tissue infections (including shingles) in patients exposed to anti-tumour necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2013; 72:229-234 [ Links ]
36. Inanc N, Direskeneli H. Serious infections under treatment with TNFα antagonists compared to traditional DMARDs in patients with rheumatoid arthritis. Rheumatol Int 2006; 27:67-71 [ Links ]
37. Doran MF, Crowson CS, Pond GR, O´Fallon WM, Gabriel SE. Frequency of infection in patients with heumatoid arthritis compared with controls. Arthritis Rheum 2002; 46:2287-2293 [ Links ]
38. Galloway JB, Hyrich KL, Mercer LK, Dixon WG, Fu B, Ustianowski AP, et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: update results from the British Society for Rheumatology Biologics Register with special emphasis on risk in the erdely. Rheumatology 2011; 50:124-131 [ Links ]
39. Greenberg J, Reed G, Decktor D. A comparative effectiveness study of adalimumab, etanercept and infliximab in biologically naive and switched rheumatoid arthritis patients: results from the US CORRONA registry. Ann Rheum Dis 2012;71:1134-1142 [ Links ]
40. Kameda H, Ueki Y, Saito K. Etanercept (ETN) with methotrexate (MTX) is better than ETN monotherapy in patients with active rheumatoid arthritis despite MTX therapy: a randomized trial. Mod Rheumatol, 2010;20:531-538 [ Links ]
41. Takeuchi T, Tanaka Y, Kaneko Y. Effectiveness and safety of adalimumab in Japanese patients with rheumatoid arthritis: retrospective analyses of data collected during the first year of adalimumab treatment in routine clinical practice (HARMONY study). Mod Rheumatol (2012) 22:327-338 [ Links ]
42. Burmester G, Mariette X, Montecucco C. Adalimumab alone and in combination with disease-modifying antirheumatic drugs for the treatment of rheumatoid arthritis in clinical practice: the Research in Active Rheumatoid Arthritis (ReAct) trial. Ann Rheum Dis 2007;66:732-739 [ Links ]
43. Singh J, Furst D, Bharat A. 2012 Update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care & Research Vol. 64, No. 5, May 2012, pp 625-639 [ Links ]
44. Calabrese LH, Zein N, Vassilopoulosen D. Safety of antitumour necrosis factor (anti-TNF) therapy in patients with chronic viral infections: hepatitis C, hepatitis B, and HIV infection. Ann Rheum Dis 2004;63(Suppl II):ii18-ii24 [ Links ]
45. Gonzales-Malaver F, Guzman-Vergara CM, Bello-Gualtero JM, Varela J, Mesa-Betancourt AM, Londoño J, et al. Latent tuberculosis infection and viral hepatitis in a Colombian cohort of patients with biological therapy. Ann Rheum Dis 2013 71:278 [ Links ]
46. Nacci F, Matucci-Cerinic M. Tuberculosis and other infections in the anti-tumour necrosis factor-alpha (anti-TNF-α) era. Best Pract Res Clin Rheumatol. 2011 Jun;25:375-88 [ Links ]
Received: November 04, 2016; Accepted: November 17, 2016











 text in
text in 


