Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Acta Médica Costarricense
On-line version ISSN 0001-6002Print version ISSN 0001-6012
Acta méd. costarric vol.53 n.4 San José Dec. 2011
Original
One Hundred Accredited Fetal Karyotypes in Costa Rica During 2009 and 2010
(Cien cariotipos fetales acreditados en Costa Rica, años 2009 y 2010)
Isabel Castro-Volio, Fernando Ortiz-Morales, Luisa Valle Bourrouet
Abstract
Objective: Identification of fetal chromosomal abnormalities is an important factor for improving perinatology and pediatric management in high risk pregnancies. The aim for this publication is to show healthcare providers, the results from our clinical research in amniotic fluid karyotype tests, obtained since they were accredited by the costarican accreditation entity, and to compare them with international standards.
Methods: Flask open cultures were conducted on 100 samples, received from January 2009 to December 2010, from social security hospitals and private health facilities and the harvest of "amniocytes" by enzyme suspension. The reasons for referral were: an abnormal ultrasound in 65% of cases, and 28% because of advanced maternal age.
Results: Fetal chromosomal disorders were found in 35%. For acceptable quantity and quality samples, a 100% culture success was obtained, with a mean 13 days response time. These data are consistent with international standards. Besides, we satisfactorily participate in annual external quality evaluations by the Cytogenetic European Quality Assessment.
Conclusion: In Costa Rica, there are perinatology services equipped with sophisticated ultrasound hardware and highly specialized personnel, so that fetal anatomical defects and other disorders rarely go unnoticed. Fetal karyotype is an essential complement for optimal clinical management of these cases, mostly when quality-guaranteed clinical trials are available.
Keywords: accreditation, prenatal diagnosis, cytogenetics, amniocentesis, Costa Rica.
For the past 40 years, in developed countries, cytogenetic fetal diagnosis is considered a routine procedure, for prenatal care of pregnancies at high genetic risk. The aims of prenatal diagnosis are a) the detection of incompatible with life or disabling fetal abnormalities, to prepare parents for the best way to receive an affected child, or to offer the option of abortion (in countries where this is legally available), b) identification of conditions that can influence the most appropriate time, place and mode of delivery to minimize fetal damage, c) the identification of fetuses that could benefit from early pediatric intervention d ) the identification of fetuses that may benefit from in utero treatment.
In Costa Rica, specifically at INISA, we´ve achieved a 24 year experience with chromosomal fetal diagnoses obtained from amniotic fluid samples, and 18 years testing blood samples from cordocentesis, to obtain the karyotype.1 We usually work with Hospitals: "R.A. Calderón Guardia" and "México", occasionally with Hospitals: "Max Peralta Jiménez", "Hospital de las Mujeres Adolfo Carit" and "Dr. Tony Facio Castro". Besides, we receive biologic samples from several private hospitals, clinics and private practice offices.
Our diagnostic tests are within the scope of our Quality Assessment System, and are also accredited as of December 9th, 2008, in accordance with Standard INTE-ISO/IEC 17025:2005 requirements "General requirements for the competition of testing and calibration laboratories". The accreditation is a process in which an authorized third party gives formal recognition of an entitys technical capability (in our case a testing laboratory) for developing a specific and perfectly defined activity. It is the mechanism that provides the necessary trust in certificates, inspection reports, test reports, calibration certificates and environmental validations issued by certification entities from different countries.2 The accreditation of our tests is for an indefinite period and is subject to annual monitoring assessments and reassessment every 4 years to a maximum of 4 years and three months, according to the evaluation and accreditation procedures of the Costarican Accreditation Entity (see accreditation scope number LE-059 on the Costarican Accreditation Entitys webpage.
Working on a management, assurance and continuous quality improvement system gives a lot of advantages to those using our diagnostic services, among others, trusting that all of their needs will be met for this subject. Our quality policy says: INISAs Senior Management is committed to ensure that tests that are part of the Quality Management System are performed sticking to good laboratory practices, using reliable and reproducible test methodologies, always using proven quality equipment and materials, as ensuring all of its suitability, requiring that key personnel gets to know quality documentation and providing training and update on matters within its competence, to guarantee valid and trustable results. It is also committed to maintain the Quality Management System on a continuous improving process. Therefore, it is committed to follow guidelines from Standard INTE-ISO/IEC 17025:2005 "General requirements for the competition of testing and/or calibration laboratories" and those from Standard INTE/ISO 15189:2008 "Clinical analysis laboratories particular requirements for quality and competition" and to fulfill legal requirements which allow and force to give quality services to its clients".
The goal for this publication is to show healthcare personnel, our results from amniotic fluid karyotype tests, obtained from the moment they were accredited and to compare them with international standards.
Materials and methods
During 2009 and 2010 we received 100 amniotic fluid samples collected from Hospitals: "México (n= 33), Calderón Guardia (n= 29), Max Peralta (n= 12), Enrique Baltodano (n= 1) and private practice facilities (n= 25). Amniocentesis to perform the fetal karyotype is a voluntary procedure, which patients orally consent. Indications to perform amniocentesis were: some type of fetal malformation shown on the ultrasound (n= 33), pregnant woman 35 years or older (n=28), fetal cystic hygroma (n=11), presence of ultrasound markers for fetal aneuploidy (n=7), fetal hydrops (n=6), oligohydramnios or polyhydramnios (n=4), positive serum marker screening (n=2) and 9 cases for less frequent indications. Ultrasound-calculated gestational age was informed for all of the amniocentesis except for one, the earliest was performed at gestational week 14, and the latest at gestational age 37. Until week 20 inclusive, 54 amniotic fluid samples were taken, after the twentieth gestational week 46 amniocentesis were performed. All samples received were satisfactory in quantity and quality except for six cases: two samples came from dead fetuses, other two samples were from fetuses with too advanced gestational ages and the other two samples were too low. Anyway, the laboratory does not reject any sample and they all enter the analytic phase. Amniocyte cultures were performed in T25 flasks, in CO2 incubators and were harvested by enzyme suspension. Banding of chromosomal preparations was made with G bands, at a resolution level between 400 and 550 bands, according to the case, and cells from at least two independent cultures were analyzed, except for cases in which the fetal karyotype resulted to be a normal male, in which the analysis from just one T25 flask was enough. At least two photographs were taken for each case.
Results
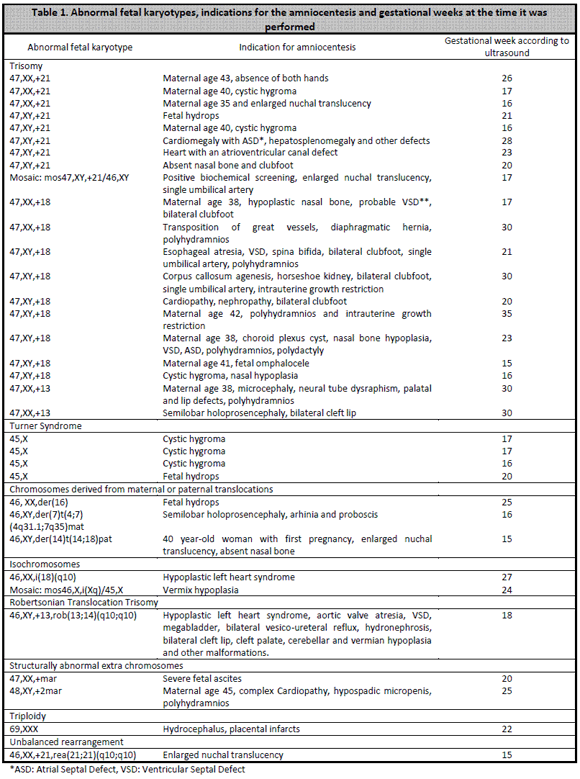
All cultures were successful, except for three cases in which there was no cell growth: a 35 weeks amniotic fluid, an (almost black) amniotic fluid from a dead fetus and another case of low fluid from a dead fetus´ hydrothorax. Therefore, culture´s success was of 100% for acceptable quality and quantity samples. Fetal karyotype was obtained in all the 97 grown samples, 30 cases were normal females, 33 cases were normal males, and there were 34 abnormal results. Characteristics from abnormal fetal karyotypes are shown in Table 1. For the 94 samples that met laboratory quality standards, response times (reported as days from the moment the sample entered the laboratory until the final report was issued) ranged between 9 days (8 cases) to 35 days (one case). Average response time was 13 days.
Discussion
European standards demand a minimum of 98% successful cultures for quality samples3 and the American College of Medical Genetics accepts a minimum of 99% success4, the same as the Association for Clinical Cytogenetics. The Association for Clinical proposes that 95% of case results should be ready within 14 calendar days,5 European standard is 21 days for 90% of samples3 and the American College of Medical Genetics says 14 days for 90% of cases,4 unless further studies are needed.
The cytogenetics laboratory fulfills international standards and guidelines in quality matters, not only regarding to time response and culture success, but also regarding to pre-analytic, analytic and post-analytic processes. For example, the necessary banding level according to the indication for the test, the cytogenetic analysis requirements for cultures harvested by enzyme suspension, the basic, moderate or extensive analysis levels in cases of possible mosaicism, etc. We also satisfactorily participate in aptitude test-like external quality evaluation rounds by the CEQA (Cytogenetic European Quality Assessment).
Regarding fetal chromosomal abnormalities (Table 1), we notice how the common denominator is the indication of an abnormal ultrasound or advanced maternal age along with ultrasound markers suggesting chromosomal abnormalities. Just one case had an enlarged nuchal translucency as the only indication for amniocentesis, and allowed to diagnose a trisomy 21. This atypical situation, was not a free trisomy 21, with 47 chromosomes, but an unbalanced rearrangement trisomy, with 46 chromosomes. This rea(21q21q) is the most common de novo rearrangements in Down Syndrome.6 Conventional cytogenetic analysis doesnt allow to distinguish if this defect is due to a robertsonian translocation or an isochromosome. When this rearrangements are molecularly studied, most of them result to be isochromosomes, derived from a single parental chromosome 21 that undergoes a U-type exchange between sister chromatids.6-8 Another prenatally diagnosed isochromosome was chromosome 18, which produced a 46-chromosome trisomy 18. This case was confirmed with QF-PCR in our laboratory, for this technique has been validated since 2008 for trisomies 21, 18 and 13.9 A third isochromosome for the long arm of the X chromosome, is a relatively frequent finding in cases of mosaic Turner Syndrome with a 45,X typical cell line. Other interesting cases were those that presented structurally abnormal extra chromosomes, also known as markers, supernumerary, accessory and B chromosomes. Some are harmless and without phenotypic consequences (B chromosomes) but others may represent a risk for the fetus. These are diagnosed in 1:1000 prenatal diagnoses, often as mosaics with a normal cell line.10 More than half the time they come from an acrocentric chromosome, which often turns to be a chromosome 15. The case with one marker and severe fetal ascites showed a small additional metacentric chromosome and the malformed fetus had two identical markers, acrocentric with satellites. By the other hand, balanced reciprocal translocations, either maternal or paternal, are common (1:500 carriers) and almost always simple, usually involve just two chromosomes, generally two autosomes and each one has just one split point. Carrier parents have a larger risk of conceiving a physically and mentally abnormal newborn, because of the segmental aneusomy phenomenon.11 For example, in our case of a mother with a (4,7) translocation, a type 1 adjacent imbalance was produced, which presents a trisomic segment for the chromosome 4 portion (q31.1→qter) and another monosomic segment for 7 (q35→qter). There is an 18,40% risk of an abnormal fetus (for any pregnancy) for the mother with this translocation, and this is a potentially viable fetus.
The cytogenetics laboratory always requests a blood sample from both parents to obtain the karyotype, unless it is a free trisomy or a monosomy X. However, we dont always get the hospitals to send us the samples or to refer these patients, so that sometimes the case study remains incomplete. This has implications for future pregnancies, as for appropriate genetic counseling it is necessary to have full information for all cases. Similarly, we request to send information about any discrepancy between the fetal karyotype diagnosed by the laboratory and the newborns phenotype, we even request a neonatal blood sample for internal quality control. Unfortunately we havent received any feedback from any healthcare center in this regard, however, the laboratory has obtained very good results on annually conducted satisfaction surveys.
In Costa Rica,
there are perinatology services equipped with very sophisticated
ultrasound
hardware and highly specialized personnel, so that fetal anatomical
defects and other disorders rarely go unnoticed. Fetal karyotype is an
essential complement
for optimal clinical management of these cases, mostly when
quality-guaranteed
clinical trials are available.
References
1. Castro-Volio I. El diagnóstico prenatal de defectos cromosómicos en Costa Rica. Rev Biol Trop 2004; 52:545-549 [ Links ]
2. Burnett D. A practical guide to accreditation in laboratory medicine. London: ABC Venture Publications, 2002. [ Links ]
3. Dagna-Bricarelli F, Hastings RJ, Kristoffersson U, Cavani S. Cytogenetic Guidelines and Quality Assurance. A common European framework for quality assessment for constitutional and acquired cytogenetic investigations. E.C.A. Permanent Working Group for Cytogenetics and Society. GUIDELINES Version 1.1 En: http://www.eurogentest.org/events/info/public/unit1/guidelines/cytogenetics/ index.xhtml [ Links ]
4. American College of Medical Genetics. Standards and Guidelines for Clinical Genetics Laboratories. 2008. En: http://www.acmg.net/AM/Template.cfm?Section =Laboratory _Standards_and _Guidelines&Template=/CM/HTMLDisplay.cfm&ContentID=6439. [ Links ]
5. Association for Clinical Cytogenetics. Professional Guidelines for Clinical Cytogenetics. Prenatal Diagnosis Best Practice Guidelines: Amniotic fluid (2005) Reformatted and updated 2007. En: www.cytogenetics.org.uk/prof.../acc_af_bp_oct2005_1.01.pdf [ Links ]
6. Schaffer LG, Jackson-Cook CK, Meyer JM, Brown JA, Spence JE. A molecular genetic approach to the identification of isochromosomes of chromosome 21. Hum Genet 1991; 86:375-82 [ Links ]
7. Schaffer LG, McCaskill C, Haller V, Brown JA, Jackson-Cook CK. Further characterization of 19 cases of rea (21q21q) and delineation as isochromosomes or Robertsonian translocations in Down Syndrome. Am J Med Genet 1993; 47:1218-22. [ Links ]
8. Chen CP, Chern SR, Tsai FJ, Wu PC, Chiang SS, Lee CC, Wang W. Down syndrome due to unbalanced homologous acrocentric rearrangements and its recurrence in subsequent pregnancies: prenatal diagnosis by amniocentesis. Taiwan J Obstet Gynecol 2009; 48:403-7. [ Links ]
9. Malespín-Bendaña W, Ortiz-Morales F, Castro-Volio I. Diagnóstico molecular de cromosomopatías fetales en Costa Rica. Acta Méd Costarric 2009; 51:236-240. [ Links ]
10. Tseng JJ, Chou MM, Lo FC, Lai HY, Chen MH, Ho ESC. Prenatal diagnosis of extrastructurally abnormal chromosomes: clinical experience and literature review. J Chin Med Assoc 2009; 72:29-33. [ Links ]
11. Gardner RJM, Sutherland GR. Chromosome abnormalities and genetic counseling. 3th ed. Oxford: Oxford Univ. Press, 2004 [ Links ]
Cytogenetics
Laboratory, INISA, Universidad de Costa Rica.
*Correspondence: Isabel Castro-Volio
E-mail: isabel.castro@ucr.ac.cr,
Sources of support: Proyecto de cooperación para la acreditación (PROCOA, Cooperation Project for Accreditation). Vicerrectoría de Investigación y Rectoría de la Universidad de Costa Rica (Research Vice Rectory and Rectory of the University of Costa Rica).
Translated by: Javier Estrada Zeledón










 text in
text in