Introduction
Cichlids are one of the higher diversity families among bony fishes with more than 200 genera and 1 700 species recognized (Nelson et al., 2016). Their geographical distribution ranges from Africa, Middle East, and India to Central and South America, these last being referred to as New World or Neotropical cichlids (Nelson et al., 2016). One factor that contributed to the high diversification of this family is the differential ability of each species to consume different food types (Axelrod & Burgess, 1979). Gross morphology examination of the digestive tract and feeding structures in cichlid fish has allowed establishing common features and trends among species, with respect to their diets, ontogeny, life histories, and ecological and phylogenetic relationships (López-Fernandez et al., 2012; Wagner et al., 2009; Zihler, 1981).
Histological and/or histochemical characterizations of the digestive tract improve knowledge about certain topics of fish biology, like trophic preferences and nutrition requirement (Rašković et al., 2011), digestive physiology (Beveridge & Baird, 2000), parasitology (de Oliveira et al., 2019), toxicology (Cengiz & Unlu, 2006; Dane & Şişman, 2020), and phenotypic plasticity (Gaucher et al., 2012; Pervin et al., 2020; Vidal et al., 2014). Considering the number of cichlids species recognized, there are few works about the histological features of their digestive tract of adult animals. To our knowledge, these are mainly focused on the African cichlids species, particularly on genus Oreochromis (Al-Hussaini, 1949; Caceci et al., 1997; Gargiulo et al., 1997; Morrison & Wright Jr, 1999; Osman & Caceci, 1991; Pasha, 1964; Scocco et al., 1996; Scocco et al., 1997; Scocco et al., 1998), and there are few works for Neotropical cichilds, (Arman & Ucuncu, 2017; da Silva et al., 2012; Hopperdietzel et al., 2014; Ramírez Espitia et al., 2020).
Gastrointestinal endocrine cells are distributed in the mucosa of the digestive tract (Banan Khojasteh, 2012). Their secretion control digestion, gut motility, enzyme secretion, nutrient absorption, among others (Volkoff, 2016). An important hormone involved in feeding behaviour is the neuropeptide Y (Npy). This peptide was described in both the brain and gastrointestinal tract of a vast number of species, acting as a powerful orexigenic neuropeptide, stimulating food intake (Matsuda et al., 2012). The number and distribution of Npy immunoreactive (Npy-ir) cells in the gastrointestinal tract vary depending on the species and the nutritional status (Hernández et al., 2018; Pereira et al., 2015; Vigliano et al., 2011). In cichlids, there is only one study analyzing the distribution of these cells in Mozambique Tilapia Oreochromis mossambicus (Pereira et al., 2017).
Cichlasoma dimerus (Heckel 1840) is a Neotropical cichlid fish that inhabits the Parana River Basin in South America (Kullander, 1983). Adults can reach 12 cm of standard length (SL) and their diet mainly consists of little aquatic insects, such as larvae of chironomidae, ephemeroptera, odonata, and trichopteran and, in lower proportion, little fishes and crustaceans (Almirón et al., 2015). In recent years, it has become an excellent animal model for different kinds of studies (Pandolfi et al., 2009), that include more than 60 research. Given the importance of the digestive tract in the overall biology of fish and the scarce information published about this topic in Neotropical cichlids, the work aims to describe anatomical, histological, and histochemical features of the digestive tract of C. dimerus, together with the immunolocalization of Npy-ir cells.
Materials and methods
Animals: C. dimerus adults of both sexes were captured in ‘‘Esteros del Riachuelo’’, Corrientes, Argentina (27°12’50’’ S & 58°11’50’’ W) and transferred to the laboratory, where they were acclimated and maintained in 130 L freshwater tanks under a 14:10 light/dark cycle and a temperature of 25 ± 2 °C for a year. Animals were fed ad libitum once a day with commercial pellets (Kilomax Iniciador, #310; Mixes del Sur, Provincia de Buenos Aires, Argentina). Fish were handled following the Principles of Laboratory Animal Care, which were approved by the Comisión Institucional para el Cuidado y Uso de Animales de Laboratorio (CICUAL), FCEyN, UBA, Argentina (Protocol #26).
Morphometric parameters and histological characterization: Adult fish (14 individuals) were randomly selected, and SL, and body weight (BW) were measured. Then, 4 h after the last feeding, they were euthanized by decapitation after anesthetized with benzocaine 0.1 %. Digestive tracts were extracted, and the intestinal lengths (IL) were measured. With those parameters, we calculated the Zihler index (ZI) and the Relative Intestinal Length (RIL) as ZI = IL/(BW^1/3) and RIL = IL/SL, according to Al-Hussaini (1949), Zihler (1981), and Karachle and Stergiou (2010). The statical comparison of BW, SL, IL, ZI, and RIL between males (N = 7) and females (N = 7) was made by different one-way ANOVA using the Infostat 2017 software (FCA, Universidad Nacional de Córdoba, Argentina). Results are expressed as mean ± SEM.
Digestive tracts were split into four recognizable regions: oesophagus, stomach, intestine, and rectum. As in fish no marked morphological distinction could be observed along the intestine (Banan Khojasteh, 2012), we divided it into equal sections for the histological process. The organs were fixed in Bouin’s solution for 24 h and they were dehydrated through crescent-gradient ethanol solutions (70°, 90°, 96°, 100°), cleared in xylene, and embedded in paraplast (Fisherbrand; Fisher Scientific, Washington, DC, USA) (Pérez Sirkin et al., 2013). The samples were cut in 7 µm coronal or parasagittal sections and mounted on gelatin-coated slides in a way that the same area of interest was available for the different histological procedures.
For histological characterization, different sections from all the collected organs were stained with modified Masson’s trichrome (mMT) and haematoxylin and eosin (H&E). The histochemical stains Alcian blue (AB) (pH = 2.5) and periodic acid-Schiff (PAS), both plus haematoxylin, were performed to identify acid or neutral glycoconjugates, respectively. The slides were examined and digitally photographed with a NIKON Microphot FX microscope.
Npy-ir cells localization: For immunohistochemistry, the samples (N = 3) obtained and cut as it was described above were deparaffinized in xylene and rehydrated in a descending series of ethanol solutions (100°, 96°, 90°, 70°), and then, in a phosphate-buffered saline (PBS) (pH 7.4). Later, endogenous peroxidase activity was blocked by incubation in 0.3 % hydrogen peroxide solution. The reduction of nonspecific staining was performed by incubation in PBS containing 5 % (w/v) non-fat dry milk at room temperature (RT). After that, the slides were maintained at 4 °C overnight with the primary anti-NPY antibody (dilution 1:2 500 in PBS, rabbit anti-porcine NPY serum, Peninsula Laboratories Inc., CA). The next day, they were washed in PBS and incubated for 1 hour with biotinylated anti-rabbit immunoglobulin (Ig) G (dilution 1:500 in PBS; Sigma-Aldrich, St Louis, MO, USA) at RT. Subsequently, peroxidase-conjugated streptavidin (dilution 1:500 in PBS; Invitrogen, Carlsbad, CA, USA) was applied for 1 hour at RT. The final reactive products were visualized with DAB Substrate Kit (Cell Marque, Rocklin, CA, USA). Then, the sections were rehydrated, slightly counter-stained with haematoxylin, and mounted with synthetic Canada balsam (Biopack, Buenos Aires, Argentina). The specificity of the antiserum was previously performed in this species with primary antisera preadsorbed with porcine NPY (Pérez Sirkin et al., 2013). Omission of first antibody control was performed.
Specific cell abundance: The abundance of Npy-ir, AB-, and PAS-positive cells were analyzed in randomly selected slides (seven slides per region) of the different organs studied. The numbers of positive cells per fold were counted manually under a light microscope, with the precaution that the same cell was not considered twice, and then, the average of each region was estimated. Results are presented qualitatively, reflecting the density of positive cells per fold.
Results
Morphometric parameters and histological characterization: The C. dimerus individuals (BW = 29.52 ± 3.18 g and SL = 8.28 ± 0.24 cm) presented a digestive tract with a short oesophagus and a blind-sac stomach. From the left side of the junction between them arose an intestine (IL = 10.81 ± 0.50 cm), with two loops in its path: on the left side, the anterior portion turned out to a cranial direction, whereas, on the right side, the second loop returned to a caudal direction concluding in the rectum and the anus (Fig. 1A). No pyloric ceca were observed in any of the animals. The mean RIL and ZI were 1.22 ± 0.07 and 3.29 ± 0.20, respectively. All the parameters evaluated did not significantly differ between males (RIL = 1.29 ± 0.07; ZI = 3.49 ± 0.17) and females (RIL = 1.19 ± 0.10; ZI = 3.20 ± 0.29).
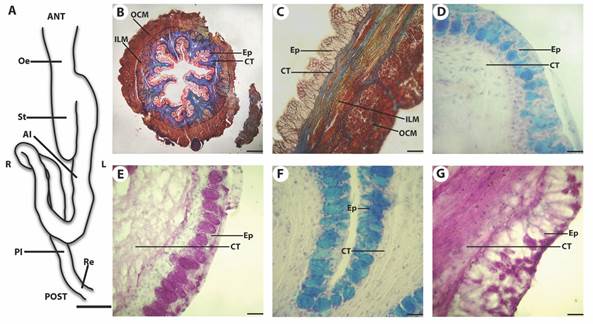
Fig. 1 A. Drawing of the ventral view of the digestive tract. Bar = 1cm. (B. C. D. E. F. and G. Microphotographs of the oesophagus of Cichlasoma dimerus. B. Oesophagus transversal panoramic view. mMT. Bar = 200 μm. C. Longitudinal section of the oesophagus. mMT. Bar = 35 μm. D. AB positive goblet cells in the anterior oesophageal mucosa. AB pH = 2.5. Bar = 20 μm. E. PAS positive goblet cells in the anterior oesophageal mucosa. PAS. Bar = 20 μm. F. AB positive goblet cells in the posterior oesophageal mucosa. AB pH = 2.5. Bar = 20 μm. G. PAS positive goblet cells in the posterior oesophageal mucosa. PAS. Oesophagus (Oe), stomach (St), anterior intestine (AI), posterior intestine (PI), rectum (Re), anterior (ANT), posterior (POST), left (L), right (R), epithelium (Ep), connective tissue (CT), inner longitudinal muscle layer (ILM), outer circular muscle layer (OCM).
Histologically, the typical four concentric tunicae of fish digestive tubes (Genten et al., 2009) were recognized in the examined organs: mucosa, submucosa, muscularis, and serosa. The oesophagus (Fig. 1B, Fig. 1C, Fig. 1D, Fig. 1E, Fig. 1F, Fig. 1G) consisted of a tunica mucosa with stratified epithelium, numerous folds (Fig. 1B, Fig. 1C), and abundant AB- (Fig. 1D, Fig. 1F) and PAS-positive (Fig. 1E, Fig. 1G) goblet cells the latter being more abundant all along the oesophagus. The AB-positive cell number increased from anterior to posterior, while the PAS-positive cell number decreased in the same direction (Table 1 and Fig. 1D, Fig. 1E, Fig. 1F, Fig. 1G). It was not possible to distinguish the lamina propria from tunica submucosa due to the absence of a continuous layer of muscular fibers. In both cases, abundant connective tissue with fibrocytes, collagenous fibers, and blood vessels was observed. No glands projecting to the connective tissue were observed. The tunica muscularis consisted of two striated muscle layers: a longitudinal inner one, with some bundles of muscle projected to the connective tissue, and a circular outer one.
Table 1 Relative abundance of Alcian Blue (AB), Periodic Acid Schiff (PAS) and Npy-immunoreactive (-ir) positive cells along the different regions of the digestive tract of Cichlasoma dimerus
| Region | ||||||||||
| Oesophagus | Stomach | Intestine | Rectum | |||||||
| Technique | Anterior | Posterior | Cardiac | Fundic | Pyloric | Anterior Posterior | ||||
| AB pH 2.5 | +++ | ++++ | - | - | - | +++ | +++ | +++ | ++ | + |
| PAS | ++++ | +++ | ∆ | ∆ | ∆ | ++ | ++ | +++ | + | + |
| Npy | NE | NE | NE | NE | NE | °°° | °° | °° | ° | - |
Note: (-) null; (NE) not evaluated; (+) low, (++) medium, (+++) high and (++++) very high density of goblet cells per fold; (∆) reactivity in the apical border of the epithelial cells; (°) low, (°°) medium and (°°°) high density of Npy-ir cells per fold.
In the junction between the oesophagus, the stomach, and the intestine, the striated muscle was gradually replaced by smooth muscle fibers. The muscle layers orientation alternated concerning that described above: the longitudinal layer became external and the circular internal in the stomach and the intestine. The oesaphagus’ epithelium was abruptly replaced with the typical epithelium of the stomach and the intestine.
The stomach (Fig. 2) was a blind sac that presented a tunica mucosa with simple columnar epithelium (Fig. 2A, Fig. 2B) and oxynticopeptic (gastric) glands in the lamina propria (Fig. 2C). The apical border of the epithelium was PAS-positive (Fig. 2D) and not goblet cells were detected. The underlying connective tissue of the tunica submucosa presented a dense bundle of collagen fibers under the epithelium and a loose one near the tunica muscularis. Two regions could be distinguished in the stomach, according to the presence/absence of gastric glands and their relative anatomical position (Fig. 2E): a glandular stomach, that is proximal, next to the oesophagus and the intestine, with the presence of gastric glands except in the most anterior portion, and a non-glandular stomach, that is in the distal region of the blind sac. The gastric glands presented polyhedral cells with a spherical nucleus, prominent nucleolus, and acidophilic cytoplasm (Fig. 2C). They formed clusters surrounded by connective tissue from the lamina propria and scattered smooth muscle fibers.
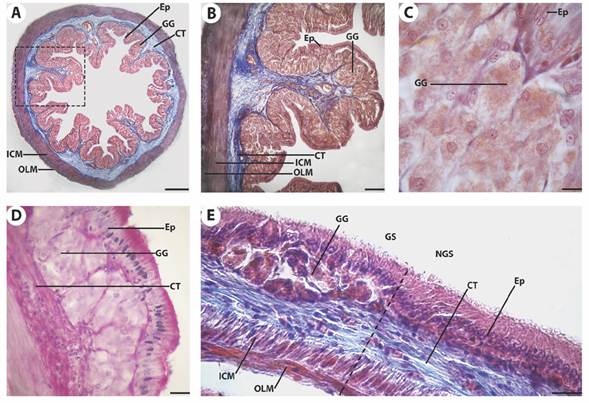
Fig. 2 Microphotographs of the stomach of Cichlasoma dimerus. A. Stomach transversal panoramic view. mMT. Bar 260 μm. B. Enlarged image from A. mMT. Bar = 100 μm. C. Gastric glands underlying the gastric epithelium. mMT. Bar = 8 μm. D. Gastric mucosa PAS positive in the apical border. PAS Bar = 20 μm. E. Longitudinal section shows the boundaries (dotted line) between glandular and non-glandular region of the stomach given by the absence of gastric glands in the latter. mMT. Bar = 20 μm. Epithelium (Ep), connective tissue (CT), gastric glands (GG), inner circular muscle layer (ICM), outer longitudinal muscle layer (OLM), glandular stomach (GS), non-glandular stomach (NGS).
Regarding the intestine (Fig. 3), modifications of the tube diameter, length and morphology of the folds, and the abundance of some cellular types were analysed. The intestinal mucosa presented a simple columnar epithelium, with enterocytes with brush border and interspersed AB- (Fig. 3C) and PAS- (Fig. 3D) positive goblet cells. These goblet cells decreased in number from anterior to posterior, with a greater abundance of AB- positive cells all along the intestine (Table 1). The PAS-positive cells slightly increase in density in the middle intestine and then decrease in the last portion of the gut. The mucosa folds decreased in number and height from anterior to posterior. In the foregut, they presented thin finger-like projections (Fig. 3A, Fig. 3B), whilst in the hindgut, they were shorter and with a foliaceous appearance (Fig. 3E). Particularly, in this region, enterocytes with large clear vacuoles in the apical cytoplasm were found (Fig. 3F). All along the intestine, the connective tissue layer of the lamina propria and the tunica submucosa was thinner than in the oesophagus and the stomach, with isolated smooth muscle fibers. The two layers of the tunica muscularis present similar width of smooth muscle fibers. The myenteric plexus between the two muscle layers were observed all along the intestine. The intestinal diameter decreased towards the posterior regions.
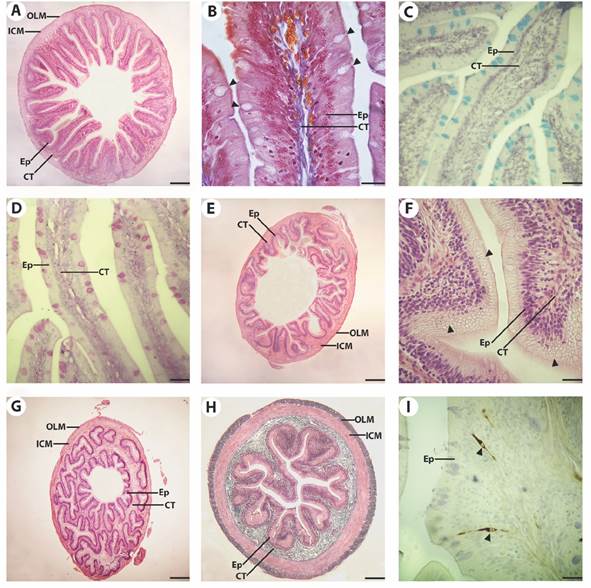
Fig. 3 Microphotographs of the intestine of Cichlasoma dimerus. A. Anterior intestine transversal panoramic view. Mucosal folds are long, finger-like projections. H&E. Bar = 200 μm. B. Anterior intestine mucosa with enterocytes with brush border and interspersed goblet cells (arrowheads). mMT. Bar = 20 μm. C. AB positive goblet cells in the anterior intestinal mucosa. AB pH = 2.5. Bar = 30 μm. D. PAS positive goblet cells in the posterior intestinal mucosa. PAS. Bar = 30 μm. E. Overview of the posterior intestine, with shorter and fewer mucosal folds. Bar = 200 μm. F. Enterocytes of the posterior region with supranuclear vacuoles (arrowheads) in the apical cytoplasm. H&E. Bar = 20 μm. G. Transition zone between posterior intestine and rectum (ileorectal valve). H&E. Bar = 200 μm. H. Coronal section of the rectum. mMT. Bar = 125 μm. I. Anterior intestinal mucosa with Npy-ir cells (arrowheads) interspersed in the epithelium. Npy IHQ + haematoxylin. Bar = 20 μm. Epithelium (Ep), connective tissue (CT), inner circular muscle layer (ICM), outer longitudinal muscle layer (OLM).
The transition between the hindgut and the rectum was abruptly marked by an ileorectal valve where thickening of the circular muscle layer, a sudden change in gut diameter, and fewer mucosal folds were observed (Fig. 3G). The rectum (Fig. 3H) presented a simple columnar epithelium with a high density of AB- and PAS-positive goblet cells. The inner circular muscle layer was thicker than the outer longitudinal.
Npy-ir cells localization: We found Npy-ir cells scattered between the enterocytes of the intestine (Fig. 3I), decreasing in density from anterior to posterior (Table 1). These cells were open-type (Barrios et al., 2020), with a narrow and elongated shape and a round nucleus. The cytoplasm was projected to the apical and basal regions, with strong immunolabel in the perinuclear zone. In the last portion of the intestine and in the rectum, no Npy-ir cells were detected in the epithelium. Besides, Npy-ir fibers into the myenteric plexus between muscle layers of the tunica muscularis were observed all along the intestine and the rectum.
Discussion
The ability of Neotropical cichlids to diversify and colonize a great variety of habitats along Central and South America has become this group in the subject of numerous studies related to their behavioral, biogeographical, ecomorphological, and phylogenetic aspects (Malabarba & Malabarba, 2020). Given that there is limited information about the digestive tube of new-world cichlids, C. dimerus has arisen as an interesting study model that could provide new perspectives on the feeding biology of this group.
The stomach’s extendible blind pouch, the left-hand exit of the stomach to the anterior intestine, and the first intestinal loop on the left side observed in C. dimerus, are considered synapomorphies within the Cichlidae family (Zihler, 1981). Also, we observed a short intestine with little coiling and low values of ZI and RIL. These morphological characteristics could be related to the dietary habits and/or the evolutionary history of the Neotropical cichlids. Regarding the first, many works have linked the gross morphology of the gastrointestinal tract with the dietary habits of a wide variety of fish species (Al-Hussaini, 1949; Karachle & Stergiou, 2010; Kramer & Bryant, 1995; Zihler, 1981). Both indexes obtained are close to the values described for omnivorous species with a preference for carnivory (Karachle & Stergiou, 2010). In this sense, C. dimerus diet in wild environments consists of microinvertebrates (Almiron et al., 2015). Concerning the phylogenetic context, many Neotropical cichlid species have lower values of ZI and RIL, with shorter intestines with little or no coiling too (Kramer & Bryant, 1995; Zihler, 1981). These species tend to be substrate-sifting invertivores, epibenthic invertebrate gleaners, and piscivores (López-Fernández et al., 2012; Montaña & Winemiller, 2013).
Some of the functions described for the oesophagus in fish have been related to food passage, digestion, and osmoregulation (Wilson & Castro 2010). In this sense, the stratified epithelium with mucus cells observed in our species could act as a mechanical and chemical barrier. The mucopolysaccharides secreted to the oesophageal lumen may help in lubrication, defence against virus and bacteria, and digestion (Genten et al., 2009; Wilson & Castro, 2010). The great abundance of mucus cells is common along the oesophagus of both Neotropical (da Silva et al., 2012; Hopperdietzel et al., 2014) and African cichlids (Morrison & Wright Jr, 1999; Okuthe & Bhomela, 2020; Scocco et al., 1998), although some differences were reported about their distribution pattern. For example, in Amatitlania nigrofasciatta, PAS-positive cells are abundant in the anterior portion; the AB-positive cells are uniformly distributed (Hopperdietzel et al., 2014). In our study PAS-positive cells present a similar distribution, but the AB-positive cells increase from anterior to posterior.
Concerning the transition zone between the oesophagus, the stomach, and the intestine, the abrupt change in the epithelial morphology and the alternate of striated and smooth muscle fibers are shared features in most of the cichlids’ alimentary tracts described so far (Ansari et al., 2020; Caceci et al., 1997; da Silva et al., 2012; Hopperdietzel et al., 2014; Morrison & Wright Jr, 1999; Okuthe & Bhomela, 2020; Pasha, 1964). Because of the presence of a thicker lamina muscularis with a high abundance of striated muscle fibers, this region may function as a pyloric sphincter, preventing that food passes directly into the intestine without being chemically digested (Ansari et al., 2020) and allowing regurgitation of food from the stomach into the oesophagus (Hopperdietzel et al., 2014).
In Neotropical cichlid species, there are some common traits in the histology of the stomach wall, such as the simple columnar epithelium with PAS-positive apical border, the absence of goblet cells, and the presence of gastric glands in all the lamina propria (da Silva et al., 2012; Hopperdietzel et al., 2014; Ramírez Espitia et al., 2020). We found these features in C. dimerus, although the blind-end of the stomach lacks gastric glands. Despite its phylogenetical proximity, in African cichlids, there are different histological arrangements of the gastric mucosa across the species. For example, gastric glands can be present throughout its extension or absent in the pyloric region, and different types and distribution of mucous glands have been reported (Ansari et al., 2020; Gargiulo et al., 1997; Morrison & Wright Jr, 1999; Okuthe & Bhomela, 2020; Pasha, 1964; Scocco et al., 1996).
We identified two distinct secretory cells in the gastric mucosa: the oxyntincopeptic and the epithelial ones with a PAS-positive apical border. The oxynticopeptic cells secrete HCl, denaturing protein and converting the pepsinogen (secreted by the same cell) into the active proteolytic form pepsin (Bakke et al., 2010). The Neutral glycoconjugates on the apical border protect the mucosa from the acid environment (Ghosh & Chakrabarti, 2015). Additionally, this secretion allows the absorption of disaccharides and short-chain fatty acids; and the mixing and emulsion of food particles with digestive enzymes (Mokhtar et al., 2017).
Along the intestine, we observed changes in the histology of the tunica mucosa related to the digestive physiology of the organ. The characteristic mucosal fold shape of the anterior region may facilitate the absorption of water-soluble molecules in the brush border and short and medium-chain fatty acids (Bakke et al., 2010). In the posterior region, enterocytes exhibit supranuclear vacuoles, which are probably due to the absorption of large peptides by endocytosis (Van den Ingh et al., 1991). Goblet cell general distribution seems to be a common feature in several carnivore species (Pereira et al., 2020), and in the omnivorous Centroamerican cichlid A. nigrofasciata (Arman & Ucuncu, 2017). It is proposed that intestinal neutral glycoproteins would be involved in the absorption and transportation of molecules through the membranes, whereas the acidic glycoproteins would be a lubricative and protective secretion (Arman & Ucuncu, 2017).
In C. dimerus, the transition between the distal portion of the intestine and the rectum is marked by an ileorectal valve, which would help to control faecal egestion. This valve was also observed in cichlids such as O. niloticus (Morrison & Wright Jr, 1999). The rectum has abundant goblet cells and a thick muscular layer, which may facilitate the egestion of faecal materials to the anus (Mokhtar et al., 2017).
Npy is considered one of the most potent orexigenic peptides in fish acting on the central nervous system (Matsuda et al., 2012; Volkoff, 2016). In C. dimerus, Npy-ir neurons localization and Npy-ir fibers abundance have been already studied (Pérez Sirkin et al., 2013). Despite its central function, the knowledge about the specific functions of this neuropeptide on the digestive system is scarce. To our knowledge, this is the first report to describe Npy-ir cells in a Neotropical cichlid species. The pattern observed in C. dimerus was also described for several species, as O. niloticus (Pereira et al., 2017), Rhamdia quelen (Hernández et al., 2018), Odontesthes bonariensis (Vigliano et al., 2011), Chanos chanos (Lin et al., 2017), Salminus brasiliensis (Pereira et al., 2015), Ictalurus punctatus (Min et al., 2009), Prochilodus lineatus (Barrios et al., 2020), Colossoma macropomum, Pseudoplatystoma reticulatum × Leiarius marmoratus (Pereira et al., 2020). The anterior portion of the intestine would be a source of peripheral signals capable of stimulating food intake when the gut is empty (Vigliano et al., 2011). Pereira et al. (2017) argued that the density of Npy-ir cells observed in the anterior intestine of several species is high because of the connection with vagal afferents that send nutritional information to the central nervous system (Olsson, 2010).
To sum up, in this work, we described the digestive tract of the South American cichlid fish C. dimerus. The gross morphology and the calculated intestinal indexes are close to those reported for omnivorous Neotropical cichlids with a preference for carnivory. Besides, the histological and histochemical characteristics found present some unique features, such as the distribution of the goblet cells and gastric glands. Considering the morphological and histological differences in the digestive tract across the cichlids, as well as the scarce information about this topic in Neotropical species, the present study provides new perspectives on this issue, in relation to feeding habits, digestive physiology, and phylogenetic context.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 


