Introduction
Antagonistic biological relationships, such as herbivory, are fundamental to understand plant-animal relationships, coevolution, and how plants can invest in different strategies to avoid consumption by animals, involving different defense mechanisms (Poore et al., 2012; Turcotte et al., 2014). Herbivory differs among terrestrial and aquatic ecosystems, being higher in the latter (Bakker et al., 2016; Cebrian & Lartigue, 2004; Reese et al., 2016). Herbivores remove on average 40-48 % of plant biomass in freshwater and marine ecosystems, which is typically 5-10 times greater than what is reported in their terrestrial counterparts (Bakker et al., 2016). In addition, herbivores affect aquatic macrophytes abundance and species composition and therefore alter the functioning of aquatic ecosystems, including primary production, biogeochemical cycling, and carbon stocks (Bakker et al., 2016; Chaichana et al., 2011). However, as much as there is a substantial literature assessing the factors influencing herbivory on macrophytes and the consequence of it on the ecosystem (see Bakker et al., 2016 for a synthesis on the subject), there is still a lack of information regarding the variables (environmental, community characteristics and/or plant functional traits) that can influence herbivory in neotropical aquatic ecosystems and how it may contribute to the high herbivory in these habitats.
Factors related to plant community structure, such as plant density and diversity, are generally used as measurement of resource quantity and may influence herbivore abundance and, consequently, the damage in plants (Kim & Underwood, 2015; Lacey et al., 2014; Otway et al., 2005; Root, 1973). There is not a clear pattern related to plant density on the herbivory amount, once they can increase the damage through changes in herbivory loads, known as resource concentration effect hypothesis (Kim & Underwood, 2015; Root, 1973) or decrease the amount of herbivory, referred as the dilution effect hypothesis (Otway et al., 2005; Wenninger et al., 2016). So far, these hypotheses have not been tested on the aquatic-terrestrial interface. Furthermore, in aquatic ecosystems, factors such as water depth and distance of macrophytes banks to the margin, as an isolation patch measurement, also may influence plant exposition and visibility to herbivores (Kolb, 2008; Korpinen et al., 2007).
In this context, plants have evolved a wide array of defense traits that provide protection against herbivory attacks, including physical traits (e.g.: presence of trichomes, spines, height, leaf toughness) and chemical compounds (e.g.: secondary metabolites, solid inclusions, hormonal responses, ergastic substances) (Agrawal & Fishbein, 2006; Parolin, 2009; Poore et al., 2012). Among physical traits, plant height and size are often related to competition and dispersal ability and are linked to environmental conditions (Schlinkert et al., 2015). However, those traits also make plants more susceptible to herbivory as those individuals become bigger and more exposed to the associated fauna, thus providing more microhabitats and food resources for them (Díaz & Cabido, 2001; Shlinkert et al., 2015).
Leaf traits are also associated to herbivory (Poorter et al., 2009; Reese et al., 2016). Leaf thickness is correlated with leaf toughness, a trait negatively associated to herbivory. Generally, thinner leaves are considered more palatable than thicker leaves (Guerra et al., 2010), because they are less tough, have less lignin and other compounds that toughen them, thus becoming easier to be consumed by herbivores (Smilanich et al., 2016). Furthermore, leaves with lower specific leaf area (SLA) are considered more resistant to herbivory, being generally avoided (Pérez-Harguindeguy et al., 2013; Poorter et al., 2009; Reese et al., 2016). SLA varies closely with other traits that can be related to herbivory defense, it can be positively related to mass-based leaf nitrogen content (which has more nutrient content, increasing the preference of herbivores), and negatively related to leaf longevity (higher leaf lifespan generally shows higher carbon investment, making leaves less palatable and less preferred by herbivores) (Pérez-Harguindeguy et al., 2013; Wright et al., 2004). Furthermore, regarding the defense system against herbivores, plants can produce a pool of toxic, deterrent, and volatile compounds (Bari & Jones, 2008; Gross & Bakker, 2012), and this reflects directly in plant physiology and in cell anatomy (Zunjarrao et al., 2019).
Thus, our aims were to assess if there is a relationship among herbivory amount and environmental variables (e.g.: water depth and distance of the margin), plant density, and plant structural traits (e.g.: height, leaf thickness, and SLA) in two Montrichardia (Araceae) species, being M. linifera and M. arborescens. We aimed to answer the following question: how environmental factors, plant density and plant traits influence plant herbivory? Our hypotheses were: i) considering the environmental factor, the herbivory amount is higher in plants occurring in deep places that generally are more distant from the margin, thus more visible; ii) concerning plant density, we expected lower herbivory in sites with dense patches, once they have more resource quantity (dilution effect); iii) regarding plant traits, we expected higher herbivory in taller plants, with thinner leaves and/or higher SLA as they are less scleromorphic and therefore, more palatable. In addition, we also aimed to indicate if there are, in both species, anatomic structures acting together with plant structural traits in the plant’s defense system, like ergastic substances and solid inclusions.
Materials and methods
Study area, sampling design and species: Sampling took place across the Curuá River, inside the Caxiuanã National Forest (FLONA-Caxiuanã), located in the municipality of Melgaço, Pará, Brazil (1°47’32.3” S & 51°26’2.5” W) near the Ferreira Penna Scientific Station, in September 2018. This side of the FLONA is characterized for having black water rivers that form a type of forest called Igapós, and for the presence of various macrophyte banks and islands.
In total, we sampled 18 sites that had Montrichardia species, and a total of 96 individuals, being 78 Montrichardia arborecens specimens, and 18 M. linifera specimens. We used a motorized boat to get to the stands where we saw the species and maintained at least 60 m of distance between surveyed locations.
Plant description: The Montrichardia Crueg (Araceae) genus consists of two species (Montrichardia linifera (Arruda) Schott and Montrichardia arborescens (L.) Schott) of large emergent plants widely distributed in the Neotropics (Croat et al., 2005), with a wide distribution in the Amazonian floodplain (Gibernau et al., 2003; Lopes et al., 2016). Individuals of Montrichardia can grow up to 6 meters in height and the populations (alone or together with other species) form rooted stands, called matupás (De Freitas et al., 2015) of varying sizes, from a few meters to large floating islands, occurring in different distances from the margin (Lopes et al., 2016). These floating islands appear with the release of a part of the macrophytes bank from the margins, the plants and sediment adhered to the roots form these islands can move in the rivers according to the water flow. Although these plants do not seem to be the most preferred hosts for some herbivores and parasites (as plant extracts show antibacterial, cytotoxic and insecticide activities), especially because of the defense mechanisms present on these organisms (e.g. chemical defense, with cells that produce alkaloids and other secondary metabolites) (Santos et al., 2014), the literature suggests these plants’ leaves and fruits are food source for fish, turtles, and big mammal such as manatees and cattle (Amarante et al., 2010). Montrichardia spp. present high phenotypic plasticity in response to the environment (Lopes et al., 2016) and participate in processes on the aquatic-terrestrial ecotone, such as stabilizing river margins (Amarante et al., 2011).
Both species of Montrichardia are frequently recorded along rivers and lakes in the Amazon basin. Montrichardia linifera is more frequently recorded along riverbanks and M. arborescens occupies a position along the floodplain and the fringe in the understory of floodplain forests (Lopes et al., 2016). The species can occur in both nutrient-rich environments, such as white-water rivers, and in nutrient-poor environments, such as the black-water Rivers, and brackish environments in the estuary of the Amazon River (Lopes et al., 2016). These species play an important role in the stabilization of river banks in the Amazon floodplains (Macedo et al., 2005).
Environmental and ecological measurements: To test the effect of environmental and ecological variables on the herbivory amount of Montrichardia we used 18 samples measured using a 1 m2 (1 x 1 m) quadrat which was placed in areas we had access to collect the species within each sampling site. In each 1 m2 quadrat we measured two environmental characteristics, i) water depth, using a graduated stick (cm) and ii) distance estimated from the plot to the riverbank using tape measure (m). Additionally, related to ecological/community factors we measured plant density, by visually assessing the percentage of cover (%) of Montrichardia species inside the 1 m2 quadrat.
Plants measurements: In each quadrat, we measured the height (cm) of each Montrichardia individual, and we selected 2 leaves of each individual to analyze the leaf traits at the laboratory. We always chose mature leaves, that were generally in the middle of the stem, thus we excluded older (more at the base of the stem) and younger (more at the apex) leaves, that could cause a bias in the data.
At the laboratory, we measured the leaf traits following the protocol proposed by Pérez-Harguindeguy et al. (2013). We measured leaf thickness (LT, mm) of each leaf in three parts (base, middle and apex) with a digital micrometer (precision of 0.01) and we took photos of each leaf to estimate leaf area and to assess the amount of herbivory on each leaf, using ImageJ, a free software. To obtain the herbivory amount, we first calculated the total area of the leaves, then the areas without herbivory damage (holes). By subtracting the area without holes from the total area, we obtained the true herbivory area on the leaf, which was then transformed into a percentage value (using the equation herbivory area (m2) * 100 / total leaf area (m2)). Posteriorly, the leaves were oven-dried at 65 °C for 72 hours, and then weighted using a digital scale (precision 0.01 g), to obtain dry mass values. We calculated the specific leaf area (SLA), using the equation SLA = leaf area (m2)/ dry mass (kg).
Anatomical analysis: To qualitatively identify anatomical structures related to plants defense, we evaluated anatomical leaves slices. For this purpose, fully expanded leaves were collected from three individuals per quadrat (N = 22), generating a total of 66 samples. The middle, midrib and apex region of the leaf blade were fixed in FAA 70 % (70º ethanol, formaldehyde and 18:1:1 v/v acetic acid), later washed in running water and dehydrated in ethylic series with alcohol 70 % for storage and to absolute ethanol to be processed (Johansen, 1940). The permanent slides were made after inclusion of the material in paraffin and sectioned transversally in a rotary microtome. The sections were stained in astra blue and aqueous safranin at 1 %, after assembled with Canada Balsam, according to the usual techniques compiled by Kraus and Arduin (1997). The anatomical analysis and the photomicrographs were taken using the photomicroscope Leica Microscope DMLB, attached to an image capture system.
The visual analysis through light microscopy was qualitative, analyzing the cell structures and compounds that can be related to plant defense, such as idioblast cells with secondary metabolites and cells with solid inclusion by comparing the structures seen in the already published papers for the Montrichardia specie and family (Amarante et al., 2015; Costa et al., 2009; Ferreira et al., 2006; Macedo et al., 2005).
Data Analyses: Prior to all hypothesis-testing analyses, we performed a Pearson Correlation test with all predictive variables, to assess for multicollinearity. No variable presented collinearity (correlations were R ≤ 0.6), so all were included on the next analyses. To test if the amount of herbivory is higher in more distant, less dense and deep patches, and in taller plants with thinner leaves and higher SLA we performed a binomial Generalized Linear Mixed Model (GLMM), for each species separately. GLMMs are fitted for data with non-normal distributions and can incorporate random effects to account for nested observations (Bolker et al., 2009; Zuur et al., 2009). For this analysis, we only used as samples individuals that were found in monospecific stands for each species, totaling 78 samples for M. arborescens and 18 for M. linifera. We included as predictors the environmental variables (water depth and distance to the margin), plant density, and plant traits (plant height, leaf thickness and SLA), and the amount of herbivory as the response variable. Since our response variable was a measure of proportion, we used the Binomial distribution family on the model (Zuur et al., 2009). At first, we build a Generalized Linear Model (GLM) using all the predictors, to perform a model selection (using Forward stepwise) based on the lowest Akaike Information Criterion (AIC) score, and thus select the best model explaining the variation in the data. After that, we built a GLMM with the fixed variables that were selected and including the plot as random effects in the model, because these factors were not part of the hypotheses but we felt could affect the results. All continuous variables were standardized. This final model was validated by checking the residuals of the analysis (Zuur et al., 2009).
We used the software R (R Core Team, 2020) to perform all analyses. For the model selection, we used the function ‘stepAIC’ in the MASS package (Venables & Ripley, 2002). We used the function ‘glmer’ in the lme4 package (Bates et al., 2015) to perform the GLMM.
Results
For Montrichardia arborescens, the densest site had 60 % of cover, while the lowest, 15 % (Mean: 28.269 ± 12.889 SD). The tallest individual was 3.40 m high, while the shortest, 0.90 m high (Mean: 130.380 ± 0.568). For the plant measurements, the highest leaf thickness value was 0.4 mm, and the lowest, 0.1 mm (Mean: 0.271 ± 0.063) and the highest SLA value was 28.004 m2 kg-1, while the lowest, 10.07 m2 kg-1 (Mean: 15.194 ± 3.787). The highest herbivory amount in an individual was 23.90 %, while the lowest was 0 (no herbivory) (Mean: 4.962 ± 5.724). For the environmental variables, the highest water depth in a plot was 155 cm while the lowest, 33 cm (Mean: 79.205 ± 31.662) and the highest distance from the margin was 50 m, while the lowest was 3 m (Mean: 13.846 ± 10.628). Furthermore, for plant density of M. linifera, the densest site had 60 % of Montrichardia cover, while the lowest, 20 % (Mean: 38.333 ± 2.832). The highest individual was 3.15 m high, while the shortest was 1.35 m high (Mean: 40.160 ± 0.471). The highest leaf thickness value was 0.4 mm, and the lowest, 0.3 mm (Mean: 0.344 ± 0.048) and the highest SLA value was 21.99 m2 kg-1, while the lowest, 8.87 m2.kg-1 (Mean: 11.962 ± 2.832). The highest herbivory amount in an individual was 10.01 %, while the lowest was 0.61 % (Mean: 2.581 ± 2.655). For the environmental variables, the highest water depth in a plot was 140 cm while the lowest, 83 cm (Mean: 103.389 ± 20.024) and the highest distance from the margin was 25 m, while the lowest was 10 m (Mean: 16.833 ± 4.176).
According to the model selection, the best model to explain the amount of herbivory on M. arborescens had the variables plant density, leaf thickness, water depth and SLA (AIC = 823.5). The GLMM results showed that, among those variables, the amount of herbivory was associated negatively with leaf thickness (Estimate = -2.866, P = 0.007, Fig. 1A) and SLA (Estimate = 0.082; P < 0.001, Fig. 1B) (Table 1). For M. linifera, the selected model had the variables plant density, leaf thickness, water depth and distance to the margin (AIC = 100.2). The results showed that, among those variables, the amount of herbivory was positively associated with leaf thickness (Estimate = 15.964, P < 0.001, Fig. 2A) and water depth (Estimate = 0.119, P < 0.001, Fig. 2B), and associated negatively with the distance to the margin (Estimate = -0.543, P < 0.001, Fig. 2C).
Table 1 Result of the Generalized Linear Mixed Models performed with variables selected by forward stepwise evaluating the relationship between herbivory in Montrichardia species and explanatory variables, in the Caxiuanã National Forest, Brazil
| Montrichardia arborescens | Montrichardia linifera | ||||||||
| Estimate | Std. Error | z | P | Estimate | Std. Error | z | P | ||
| Intercept | -0.669 | 0.695 | -0.962 | 0.336 | Intercept | -12.438 | 2.582 | -4.816 | < 0.001 |
| Plant Density | -0.018 | 0.016 | -1.151 | 0.250 | Plant Density | -0.007 | 0.024 | -0.290 | 0.772 |
| Leaf Thickness | -2.866 | 1.069 | -2.682 | 0.007 | Leaf Thickness | 15.964 | 2.499 | 6.388 | < 0.001 |
| Water Depth | -0.001 | 0.006 | -0.194 | 0.846 | Water Depth | 0.119 | 0.036 | 3.346 | 0.001 |
| SLA | -0.082 | 0.017 | -4.725 | < 0.001 | Distance to the margin | -0.543 | 0.141 | -3.847 | < 0.001 |
| Random Effects | Random Effetcs | ||||||||
| Variable | Variance | Std. Dev. | Variable | Variance | Std. Dev. | ||||
| Plot | 0.441 | 0.664 | Plot | < 0.001 | < 0.001 | ||||
Values in bold indicate significant relationships.
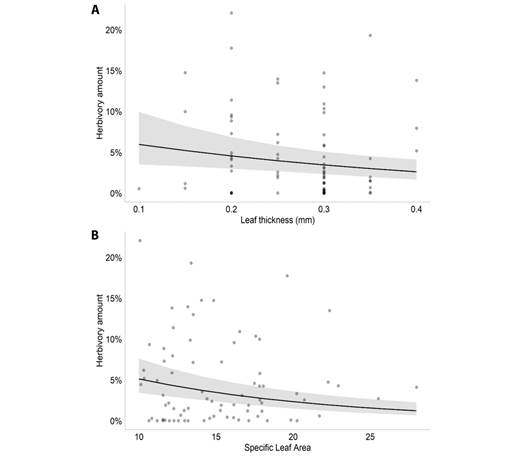
Fig. 1 Plot of the significant effects of the Generalized Linear Mixed Model performed for M. arborescens in the Caxiuanã National Forest, Brazil. A. Effect of leaf thickness (mm) on leaf herbivory amount of Montrichardia arborescens; B. Effect of specific leaf area on leaf herbivory amount of Montrichardia arborescens.
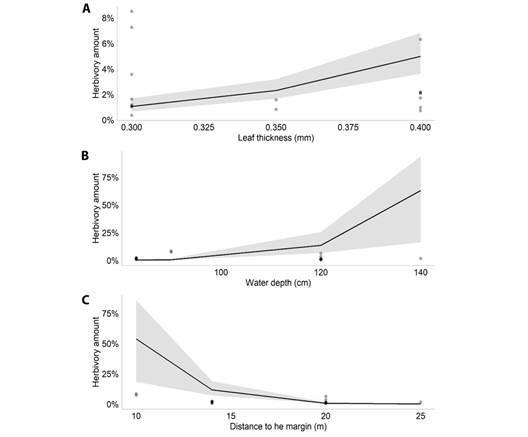
Fig. 2 Plot of the significant effects of the Generalized Linear Mixed Model performed for M. linifera in the Caxiuanã National Forest, Brazil. A. Effect of leaf thickness (mm) on leaf herbivory amount of Montrichardia linifera; B. Effect of water depth on leaf herbivory amount of Montrichardia linifera; C. Effect of the distance to the margin on leaf herbivory amount of Montrichardia linifera.
In relation to leaf anatomy (for both species), leaves were dorsiventral with stomata present only on the abaxial epidermis (Fig. 3). The epidermal contended a single cell layer with thick anticline and periclinal walls covered with an evident cuticle (Fig. 3A). We found anatomic and chemical structures related to defense, such as the idioblast cells with phenolic compounds and cells with solid inclusions - druses, like calcium oxalate crystals (Fig. 3B, Fig. 3C, Fig. 3D, Fig. 3E).
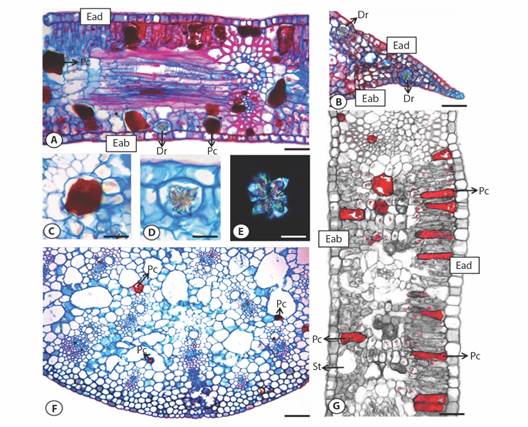
Fig. 3 Cross section of Montrichardia spp. leaves: A. leaf blade - M. linifera; B. leaf apex - M. linifera; C. phenolic idioblasts - M. arborescens; D. druse - M. linifera; E. druse under polarized light - M. linifera; F. midrib - M. arborescens; G. leaf blade - M. arborescens. Dr: druse, Pc: phenolic compounds, St: stomata, Eab: abaxial epidermis, Ead: adaxial epidermis. Bars: C. D. and E. = 15 µm, A. B. and G. = 50 µm, F. = 100 µm.
Solid inclusions were observed both in mesophyll and in midrib parenchimal cells. A high number of druses were found inside idioblasts in the leaf apex, both in the palisade and spongy layers. The idioblast cells with phenolic compounds were found in midrib (Fig. 3F). The phenolic compounds were present in leaf in the palisade and spongy tissues, also along the cortical and parenchimal region in the midrib (Fig. 3F). The phenolic idioblasts have a varied shape, occupying a large part of the tissues (rounded to elongated) (Fig. 3G).
Discussion
According to our results, our hypothesis was partially corroborated, in which the environmental factors and plant traits explained the observed herbivory amount in Montrichardia sp. Contrary that we predicted, the plant density was not related to the herbivory amount in the studied species. For M. arborescens the leaf traits, such as leaf thickness and SLA, were related to the herbivory amount. While in M. linifera both leaf traits, such as leaf thickness, and environmental factors, as water depth and distance to the margin, were related to the herbivory amount.
Among plant traits that were measured, in both species leaf thickness was related to herbivory amount, however in M. arborescens it was associated negatively and in M. linifera the proportion of herbivory was positively associated with leaf thickness. The negative relationship that we observed in M. arborescens can be associated with the fact that leaf thickness is related to structural defense, in which thicker leaves tend to have greater structural resistance against leaf-chewing herbivores (Onoda et al., 2011). Thus, plants can produce thicker leaves to defend themselves against their herbivores. Muiruri et al. (2019), found that leaf thickness had a significant effect on herbivory load, reducing gall abundance in thicker leaves. Furthermore, they showed that physical traits might be more important determinants of plant herbivory than nutritive and chemical defense traits. Additionally, plant traits, such as the leaf thickness, can affect the transmission of vibrational signals and cues that can be used by animals to plant detectability. Insects, as the caterpillars, can use plant-borne vibrations to extract and use information from their biotic and abiotic environment (Velilla et al., 2020), influencing in the herbivory load. For example, plants can evolve thicker leaves to avoid herbivores, however, they may reduce effective use of vibratory cues by the predators and parasites that can eat these herbivores (Velilla et al., 2020). This can be one hypothesis to explain a positive relation between herbivory load and leaf thickness, as we observed in M. linifera. We were not able to identify the herbivore species that were damaging Montrichardia individuals, but on more than one occasion we saw caterpillars and eggs on the leaves (personal observation).
The SLA was also negatively related to the herbivory load in M. arborescens. SLA is generally positively related to plant growth rate and leaf quality (Milla et al., 2008), in which low SLA values indicate a high investment in carbon to build leaves and higher leaf longevity (Wright et al., 2004). There are some interspecific herbivory patterns in diverse land communities related to functional traits, in which, in some communities, leaves with lower SLA are less eaten by herbivores (Poorter et al., 2009; Reese et al., 2016). However, we observed a negative relationship between SLA and herbivory, in which leaves with higher carbon investments were more eaten. This possibly can be associated to higher leaf longevity (Pérez-Harguindeguy et al., 2013; Wright et al., 2004), being exposed to herbivores for more time than leaves with short lifespan.
Insect herbivory patterns are influenced by multiple ecological drivers acting at different spatial scales (Wang et al., 2022). In M. linifera the herbivory amount was negatively associated with the bank distance to the river margin. This patch distance can be viewed as an isolation measurement, because fragmented populations are less visible and less apparent to insect pollinators, moreover, visitors (including grazers and pollinators) (Kolb, 2008). Isolation is considered an important factor affecting insect herbivory, in which, insect herbivores tend to reach higher densities in patches that can be accessed and colonized more easily (Wang et al., 2022). The patch isolation can also decrease insect herbivory via changes in abiotic factors, such as humidity, wind and temperature, and resource limitation (i.e. “bottom-up” factors) that affect herbivores directly (Maguire et al., 2016).
The herbivory amount in M. linifera was positively associated with water depth. Resource availability (nutrients, light) declines exponentially with increasing depth (Bakker et al., 2016). The water motion and light levels also declines, reducing photosynthesis and nutrient uptake rates, making these areas more vulnerable to being consumed by animals, increasing grazing efficiency and herbivore density (Korpinen et al., 2007). It is possible that the herbivory amount in M. linifera is increasing because of these factors (although the range of water depth found in our study is not that high - 140 cm to 83 cm), which could indicate that the species that is inhabiting a more ‘resource limiting environment’ are more susceptible to herbivore damage.
Among the strategies, some of these mechanisms are compartmented inside the plant (Giordano et al., 2020), like chemical compounds (i.e. secondary metabolites, solid inclusions, hormonal responses, ergastic substances) (Agrawal & Fishbein, 2006; Carmona et al., 2011), to avoid being consumed by animals. As anatomical defense structures, the idioblast cells with phenolic compounds seen in both Montrichardia species play a central role in this group, including acting as a barrier to several pathogens that could prevent infection diffusion, penetration of fungi, defense against various bacterial and damage caused by insects or by grazing animals, like herbivores (Liang et al., 2007; Zhang et al., 2019). Phenols are aromatic compounds derived from the shikimic acid pathway and also protect cells from UV-B radiation and oxidative stress (Berli et al., 2010; Uleberg et al., 2012). The phenolic compounds seen in the idioblasts along the Montrichardia leaf blade corresponding to substances in the flavonoid group (Amarante et al., 2015), these phenolic compounds have pharmacological and anti-nutritional action, inhibiting lipid oxidation and fungal proliferation (Soares, 2002). The plant normally uses these phenolic compounds as an antiseptic and to protect itself against dehydration, rot and attack by animals (Ferreira et al., 2006). Our phenolic structures are similar to those found by Amarante et al. (2015).
Allied to that, solid calcium inclusions are widespread in mesophyll and in midrib parenchymal cells for both Montrichardia species. Calcium oxalate crystals is the most prominent storage of calcium salts (Fahn, 1990) and in leaf blades it protects plants from biotic and abiotic stresses (Giordano et al., 2020). These specialized cells are responsible for metal detoxification (Franceschi & Nakata, 2005), light scattering (Gal et al., 2012), high-capacity calcium (Ca) regulation, can sequester heavy metals ions and mainly protection against herbivores, for being a multifaceted roughly spherical structure that is unpalatable to herbivores (Giordano et al., 2020; Pierantoni et al., 2018). The presence of calcium oxalate crystals is one of the outstanding characteristics of the Araceae family (Costa et al., 2009; Ferreira et al., 2006), seen by Amarante et al. (2015). One hypothesis considered as the cause of the toxicity on those plants from these family is the fact that these crystals, in the form of druses or raffids, are associated with toxic substances and are found in the latex expelled by the plant (Martins et al., 2005). In humans, the latex with druse content causes burns, rashes and spots on the skin and in contact with the eyes can even cause blindness (Amarante et al., 2011; Macedo et al., 2005).
Thus, these two advantageous structures of cellular metabolism, idioblasts cells with phenol and solid inclusions (like druses) on both Montrichardia species, are all part of the plant defense system and it is influenced by various genetic and environmental factors, as seen in other species from the Araceae family (Ferreira et al., 2006) and Montrichardia species (Amarante et al., 2011; Macedo et al., 2005). Nutrient availability is a determinant in the allocation of metabolites for defense against herbivores (Izzo et al., 2018). Overall, these strategies are considered anti-herbivore defenses in freshwater macrophytes and seagrassess (Bakker et al., 2016), making leaves with low palatability to keep pathogens, from bacteria and fungi up to insects and other herbivores, away (Giordano et al., 2020). This mean the plant’s defense mechanisms are effective, probably acting immediately after an attack and preventing severe damages (induced response), or only sustaining damage from specialist herbivores that evolved to overcome those defenses (Ali & Agrawal, 2012; Mithöfer & Boland, 2012), or a combination of both (Ali & Agrawal, 2012).
Concluding, our results indicate that herbivory in Montrichardia species could be explained by combination of environmental (patch isolation and depth water) and plant traits. We found that leaf traits were important factors that drive changes in herbivory load, mainly leaf thickness and specific leaf area. Furthermore, Montrichardia species invest in chemical compounds and solid inclusions to avoid severe damage on the leaves, thus may sustain less damage than other macrophyte species. Our findings bring new information regarding which set of variables explain the herbivory amount in aquatic macrophytes, emphasizing the importance of landscape isolation, leaf traits and defense compounds on those organisms in freshwater ecosystems.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 


