Introduction
Snakebite envenoming is a global public health problem that, despite its complexity and magnitude, has not received enough attention from health authorities, pharmaceutical companies and research agencies in all parts of the world (Gutiérrez, Williams, Fan, & Warrel, 2010). Therefore, the World Health Organization (WHO) recently included snake bite envenoming on its list of neglected tropical diseases (Gutiérrez et al., 2017).
This environmental and occupational disease affects mainly agricultural workers in rural communities in tropical regions, afflicting almost exclusively the impoverished population (Williams et al., 2010). The victims of envenoming generally live in remote communities, far from medical facilities, thus lacking timely medical attention. Furthermore, many people die on the way to medical centers, and others mostly rely on popular medicine and, therefore, are not included in the official hospital records (Otero-Patiño, 2009).
Between 130 000 and 150 000 snakebite cases are recorded in the Latin American region each year, with an estimated 2 300 annual deaths (Chippaux, 2006). Panama has the highest incidence of snakebites in the region, registering 54-62 cases per 100 000 inhabitants (approximately 2 000 bites per year). The mortality rate is estimated at 0.54/100 000 inhabitants (Ministry of Health, 2011). Many affected people develop physical and psychological sequelae and permanent disability, which is estimated to exceed the number of deaths (WHO, 2010; Gutiérrez et al., 2017).
In Latin America, snakes of the genus Bothrops inflict the greatest number of accidents, and B. asper is the principal species responsible for envenomings in Southern Mexico, Central America, and Northern South America. In the Central American region, it is estimated that B. asper is responsible for approximately 50-80 % of snakebites, as well as most of the deaths due to these envenomings (Otero-Patiño, 2009). Popularly, B. asper is known as “Terciopelo”, “Barba amarilla”, “nauyaca” or “equis”. It is widely distributed in the humid lowlands of Mexico, Central America, Venezuela, Colombia and Ecuador, living both in forests and in areas of livestock and agricultural use, which consequently increases the likelihood of contacts between snakes and workers or their homes (Otero-Patiño, 2009).
The venom of B. asper induces both local alterations at the site of venom injection (edema, hemorrhage, dermonecrosis, flictenas, myonecrosis), and systemic effects that can be life threatening (defibrination, bleeding distant from the bite site, nephrotoxicity and cardiovascular shock) (Otero et al., 2002; Gutiérrez & Lomonte, 2003; Gutiérrez, Escalante, & Rucavado, 2009; Gutiérrez, Rucavado, Chaves, Díaz, & Escalante, 2009). Snake venom is a complex mixture, which in addition to a large variety of toxins, enzymes and proteins without enzymatic activity, also contains amino acids, nucleotides, phosphorylated sugars, lipids, and metal ions. Research in proteomics has shown a high number of different proteins in the venom of B. asper (Alape-Girón et al., 2008), which predominantly belong to the families of metalloproteinases (41-44 %), phospholipases A2 (29-45 %), serine proteinases (4-18 %), L-amino acid oxidases (5-9 %), disintegrins (1-2 %) and lectin-like C-type proteins (Angulo & Lomonte, 2009).
There is significant qualitative and quantitative variation in the biochemical composition of venoms, both between and within species. These differences have their origin in ontogenetic, geographic, phylogenetic, and environmental factors, as well as individual factors (Gutiérrez, Chaves & Bolaños, 1980; Saldarriaga et al., 2003; Núñez & Otero, 1999; Alape-Girón et al., 2008).
Martínez (1983a); Martínez (1983b) and Quintero, González, Suárez, & Arantes (2007) studied some biochemical and toxicological characteristics of B. asper venoms from Panama. A previous study described slight variations in the toxic and enzymatic activities in the venoms of pools of B. asper venoms from several regions in Panama, although venoms were in general similar (Vélez et al., 2017).
Antivenoms remain the only specific and effective treatment for envenoming caused by snakebites (Gutiérrez, Williams, Fan, & Warrell, 2010). Owing to the described variation in venom composition, antivenoms should be subjected to preclinical evaluations (neutralization of lethality and other toxic activities) against the most relevant snake venoms from a particular country or region (Otero et al., 1995; Williams et al., 2010; Gutiérrez et al., 2013). To this end, the World Health Organization (WHO) has recommended the preparation of reference national venom pools of the medically-relevant snake species and to characterize such reference venoms, in order to use them in the preclinical evaluation of antivenoms being used in the countries.
The venom of B. asper from various countries has been extensively studied in terms of its biochemical and toxicological characteristics (Angulo & Lomonte, 2009; Gutiérrez, Escalante, & Rucavado, 2009; Gutiérrez, Rucavado, Chaves, et al., 2009). However, no national reference venom of B. asper has been prepared and characterized in any country. The present study was carried out with the aim of characterizing the toxicological and enzymatic effects, as well as the immunochemical reactivity, of a reference venom of B. asper from Panama, prepared by generating a pool of venoms from four geographic regions in this country. This venom mixture can be used in the preclinical evaluations to test the efficacy of antivenoms distributed in Panama.
Materials and methods
Animals and venom: The venom was obtained via manual milking of 78 adult specimens of B. asper collected in the following four geographical regions of mainland of Panama: Zone 1: Bocas del Toro, Veraguas’ Caribbean region, Colon and Guna Yala; Zone 2: Chiriquí and Veraguas’ Pacific region; Zone 3: Los Santos, Herrera and Coclé; and Zone 4: Panama Oeste, Panama and Darien (Fig. 1). The snakes were kept in captivity at the Serpentarium located in the School of Biology at the University of Panama. Once collected, the crude venom was centrifuged for 15 min at 3 000 rpm. The supernatant was frozen at -20 °C, lyophilized and stored at -20 °C until use. To ensure geographical representation, a homogeneous mixture was prepared using 100 mg of lyophilized venom from each geographical zone in order to prepare the reference venom. Snakes used for the preparation of this reference venom were adults; 52 % were females and 48 % were males. Approximately half of the specimens were collected during the dry season (December to April) and the other half during the rainy season (May to November).
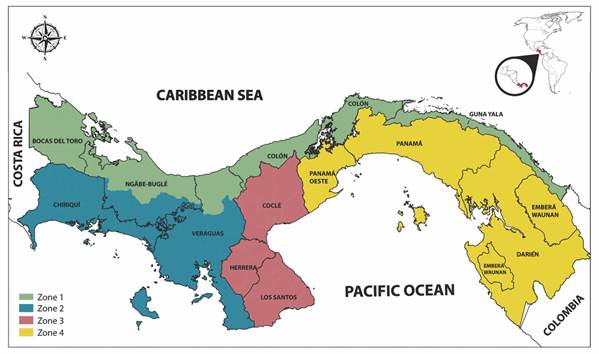
Fig. 1 Geographical regions where Bothrops asper snakes were collected. Zone 1: Bocas del Toro, Veraguas’ Caribbean region, Colon and Guna Yala; Zone 2: Chiriquí and Veraguas’ Pacific region; Zone 3: Los Santos, Herrera and Cocle; and Zone 4: West Panama, Panama and Darien.
For the in vivo tests, mice of the strain CD-1, with a body weight of 18-20 g, were used following the recommendations of the Guide for the Care, Ethics and Use of Laboratory Animals of the National Research Council of the National Academies (2011). The mice were provided by the Bioterium at the University of Panama. When these studies were carried out, the Ethics Committee in Animal Use had not been established in our country.
Venom toxicological and enzymatic effects
Hemorrhage: Groups of four mice per dose received intradermal (i.d.) injections, in the ventral abdominal area, of a range of doses of venom (1.0-16.0 μg) in a volume of 100 μl of phosphate buffered saline solution (PBS) at pH 7.2. The control group received an equivalent volume of PBS. The animals were sacrificed 2 h later by inhalation of anesthetic (Sevorane®) and the area of hemorrhage in the inner side of the skin was measured. The Minimum Hemorrhagic Dose (DHm) was the dose of venom that induced an area of hemorrhage of 10 mm in diameter, following the method of Kondo, Kondo, Ikesawa, Murata, & Ohsaka, (1960), modified by Gutiérrez, Gené, Rojas, & Cerdas (1985).
Edema-forming: Groups of four mice per dose received subcutaneous (s.c.) injections, in the right paw (subplantar), of a range of doses of venom (0.031-0.5 μg), diluted in a volume of 5 μl of PBS. As a control, 5 μl of PBS were injected into the left paw of each mouse. After 3 h, the relative volume (edema) of the right paw versus the left paw (control) was measured in each animal, using an LE 7500 digital plethysmometer from PanLab Harvard Apparatus. The Minimum Edema-forming Dose (DEm) was the dose of venom that caused 30 % increase in paw volume at 3 h, following the method of Yamakawa, Nozaki, & Hokama, (1976), modified by Chaves, Barboza, & Gutiérrez (1995).
Myotoxicity: Groups of four mice per dose were received intramuscular (i.m.) injections, in the right gastrocnemius, of a range of doses (25 to 50 μg) of B. asper venom in 100 μl of PBS. The control group received the same volume of PBS. After 3 h, mice were anesthetized and blood samples were collected from the orbital venous sinus, using heparinized capillaries, to quantify the activity of the enzyme creatine kinase (CK) in the plasma, with a commercial kit (CK NAC liquiUV, Human). The Minimum Myotoxic Dose (DMm) was the dose of venom that induced a four-fold increase in plasma CK activity, compared with the control group (Gutiérrez, Arroyo, & Bolaños, 1980). Myotoxic activity was also expressed as the CK activity of plasma from mice injected with 50 μg of venom (Segura et al., 2010).
Lethality: Groups of five mice per dose received intraperitoneal (i.p.) injections of a range of doses of venom (1 to 7 mg/kg), diluted in a volume of 500 μl of PBS. The number of mice dead at 48 h was recorded. Lethal Dose (LD50) was obtained by the Spearman-Karber method (WHO, 1981), using TOXICALC software provided by the Instituto Clodomiro Picado (University of Costa Rica).
Coagulant effect: Test tubes with 200 μl of citrated platelet-free human plasma were incubated at 37 °C with a volume of 100 μl of PBS containing different amounts of venom (0.16-1.8 μg). The clotting time was recorded in seconds. The Minimum Coagulant Dosage (DCm) was the dose of venom that induced the coagulation of the plasma in 60 s (Gené, Roy, Rojas, Gutiérrez & Cerdas, 1989).
Defibrinating effect: Groups of four mice received intravenous (i.v.) injections, into the caudal vein, with different doses of venom (0.625-20.0 μg) in a volume of 200 μl of PBS. After 1 h the mice were anesthetized by inhalation of Sevorane® and cardiac puncture was realized to obtain whole blood samples, which were placed in glass tubes and maintained at room temperature for 1 h. The Minimum Defibrinating Dose (DDm) was the minimum dose of venom that caused anticoagulation in all injected mice (Theakston & Reid, 1983; Gené et al., 1989).
Proteolysis: Proteolytic activity was tested by incubating various amounts of venom in 2 ml of casein (2 % in PBS, pH 8.0) for 30 min. The reaction was stopped by adding 4.0 ml of 5 % trichloroacetic acid. After 5 min, the tubes were centrifuged at 3 000 rpm for 10 min and the absorbances of the supernatant were recorded at 280 nm against the blank reagent. The Minimum Proteolytic Dose (DPm) was determined as the amount of venom that produced an absorbance change of 0.5 at 280 nm (Lomonte & Gutiérrez, 1983).
Indirect Hemolysis (Phospholipase A2 Activity): The activity of phospholipase A2 was determined using agarose plates (0.8 % in PBS, pH 7.2) prepared with human erythrocytes, CaCl2 (0.01 M) and a suspension of egg yolk (1:4 in PBS). Volumes of 15 μl of solution with increasing concentrations of venom (1.2-30.0 μg) were applied in 3 mm diameter wells. Then, the plates were incubated for 20 h at 37 °C and the diameter of hemolytic halos was measured. The Minimum Hemolytic Dose (DHLm) was the dose of venom that induced a hemolysis halo of 20 mm diameter in 20 h (Gutiérrez, Avila, Rojas & Cerdas, 1988).
Immunochemical characterization of the venom
Quantification of proteins: The protein content of the venom was estimated by the Bradford method, as modified by Spector (1978), using bovine serum albumin as the standard.
Electrophoresis-SDS PAGE: Electrophoresis was performed in 15 % polyacrylamide vertical minigels in the presence of sodium dodecylsulfate (SDS-PAGE) (Laemmli, 1970). For each run, 15 μg of venom equivalent to 11 μg of protein were used under reducing conditions with β-mercaptoethanol, and 150 V were applied for 70 min in a Mini-Protean II chamber from Bio-Rad (Richmond, CA, USA). The reference venom (pool of the four regions) and venoms from each region were run in parallel with a molecular mass marker (Bio-Rad-catalog No. 161-0304). After separation, gels were stained with Coomassie Brilliant Blue. Densitometry was performed using myImageAnalysis™ Software from Thermo Fisher Scientific.
Preparation of anti-B. asper serum: Two New Zealand female rabbits (2-3 kg) were immunized using variable doses of the reference venom from Panama, ranging between 250 and 1 000 μg, which were applied s.c. (the first two doses) and i.m. (the last dose), at monthly intervals, using appropriate adjuvants (complete and incomplete Freund’s adjuvants). After 60 days, blood was collected, and the serum was separated by centrifugation and passed through protein A columns to obtain IgG (specific and non-specific against the venom). The fractions collected were dialyzed against distilled water, then lyophilized and frozen until use (Lomonte, 2002).
Immunological reactivity (Western blotting): The reference venom from Panama was separated by SDS-PAGE (as previously described) and transferred for 2 h to nitrocellulose membranes in a Mini-Transblot® chamber from Bio-Rad.
As a primary antibody, polyvalent antivenoms from different regions of Latin America were used: Argentina (Instituto Biológico Argentino Saic-lot 1604), Brazil (Instituto Vital Brazil SA, lot 095106E), Colombia (Laboratorio Probiol SA, lot Ap310ix10), Costa Rica (Instituto Clodomiro Picado, lot 4691110POLQ) and Panama (anti-B asper serum produced in rabbits).
The membranes were incubated at room temperature with the corresponding antivenoms in a dilution of 1:1 000 (in PBS Tween 20) for 3 h. They were then incubated for 2 h at room temperature with a dilution 1:5 000 of anti-horse IgG or anti-rabbit IgG (whole molecule)-Peroxidase antibody produced in rabbit, as a secondary antibody. Subsequently, the reaction was revealed using a solution of hydrogen peroxide in the presence of 4-Cl-1-naphthol and diaminobenzidine. The reaction was stopped by adding distilled water at the time the bands appeared.
Presentation of the results: All toxicological and enzymatic tests were performed in triplicate. The results are expressed as the mean ± S.E.M (standard error of the mean), except for lethality, in which case variability is expressed with 95 % confidence intervals.
Results
The reference venom of B. asper from Panama induced characteristic local and systemic effects of venoms of the Viperidae family. This venom possesses lethal, hemorrhagic, myotoxic, edema-forming, defibrinating and in vitro coagulant activities; it also shows proteolytic and phospholipase A2 enzymatic activities (Table 1). In addition to estimating the Minimum Myotoxic Dose, creatine kinase (CK) activity 3 h after the intramuscular injection of 50 μg of venom corresponded to 10 196 ± 1 817 U/l.
Table 1 Toxicological and enzymatic activities of the reference venom of Panamanian Bothrops asper
| Test | Results ± S.E.M. |
| Hemorrhagic activity (DHm) | 4.93 ± 0.29 μg |
| Myotoxic activity (DMm) | 10.2 ± 1.68 μg |
| Edema-forming activity (DEm) | 0.32 ± 0.04 μg |
| Coagulant activity (DCm) | 0.53 ± 0.04 μg |
| Defibrinating activity (DDm) | 1.25 ± 0.0 μg |
| Proteolytic activity (DPm) | 1.39 ± 0.1 mg |
| Phospholipase A2 activity (DHIm) | 2.93 ± 0.3 μg |
| Lethal activity (DL50) | 4.84 mg/kg (3.98-5.88) |
DHm: Minimum Hemorrhagic Dose; DMm: Minimum Myotoxic Dose; DEm: Minimum Edema-forming Dose; DCm: Minimum Coagulant Dose; DDm: Minimum Defibrinating Dose; DPm: Minimum Proteolytic Dose; DHlm: Minimum Indirect Hemolytic Dose (Phospholipase Activity A2); DL50: Medium Lethal Dose. The 95 % confidence intervals are shown in parentheses. S.E.M.: Standard Error of the Mean. The definitions of each dose are detailed in the Materials and Methods section.
When venom protein components were separated by SDS-PAGE (Fig. 2), fractions with molecular weights between 8 and 70 kDa were observed, with the presence of predominant bands at 70 kDa, 42-48 kDa, 24-37 kDa, 15 kDa and 11 kDa. The percent composition of the main protein fractions in Panamanian reference venom can be seen in Table 2.
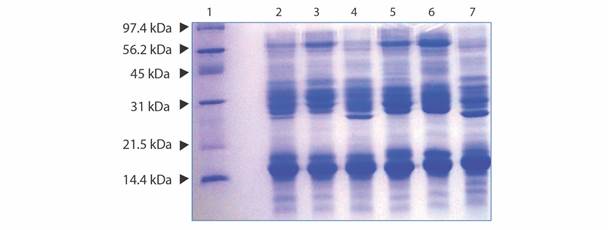
Fig. 2 Electrophoretic patterns obtained by SDS PAGE (15 % acrylamide) run under reducing conditions using 15μg of Panamanian B. asper reference venom, Costa Rican venom and venom from each Panamanian zone. Lane 1: Low Range Molecular Weight Marker (Bio-Rad); Lane 2: Reference B. asper venom from Panama; Lane 3: B. asper venom from Costa Rica; Lane 4: B. asper venom from Panama Zone 1 (Atlantic); Lane 5: B. asper venom from Panama Zone 2 (Chiriquí and Pacific Veraguas); Lane 6: B. asper venom from Panama Zone 3 (Los Santos, Herrera and Cocle); Lane 7: B. asper venom from Panama Zone 4 (Panama and Darién). Gels were stained with Coomassie Brilliant Blue.
Table 2 Percent composition of the protein fractions of Bothrops asper venom from Panama
| Molecular Weight (kDa) | Content (Average percent) |
| 55-70 | 7.1 |
| 42-48 | 8.3 |
| 24-37 | 37.4 |
| 15 | 40.0 |
| 11 | 7.2 |
Determined by densitometry using My Image Analysis™ software (Thermo Scientific).
Fig. 3A shows the SDS-PAGE profile of the proteins of B. asper reference venom from Panama. Western blot analysis of the immunological reactivity of several Latin American antivenoms against the reference venom of B. asper from Panama is shown in Fig 3B. All antivenoms reacted against most of the bands, but a higher reactivity was observed for the antivenoms of Costa Rica and Colombia.
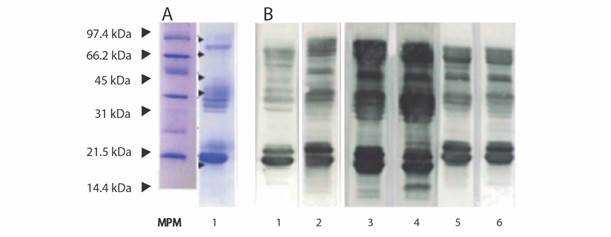
Fig. 3 Electrophoresis and Western blotting assays. A. SDS PAGE electrophoresis of B. asper reference venom from Panama. MPM: Low Range molecular marker (Bio-Rad); 1. Panamanian B. asper reference venom (15 μg of lyophilized venom) separated by SDS PAGE (15 %) under reducing conditions; B. Immunological reactivity against B. asper reference venom from Panama determined by Western blotting using antivenoms produced in Latin America. 1. Antivenom anti B. asper from Panama (produced in rabbits); 2. Antivenom from Venezuela; 3. Antivenom from Costa Rica; 4. Antivenom from Colombia; 5. Antivenom from Brazil; 6. Antivenom from Argentina.
All antivenoms showed immunorecognition for protein bands of 15-16 kDa and 25-30 kDa, the former bands containing PLA2, such as myotoxins, and the latter bands containing metalloproteinases PI, serine proteinases and PLA2. Fractions of proteins with masses between 42-48 kDa and 55-70 kDa were highly recognized by all antivenoms. All antivenoms, but particularly those from Costa Rica and Colombia, presented immunoreactivity against the 11 kDa fraction.
Discussion
This study follows the recommendation of the WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins (WHO, 2010) in the sense that it is relevant to prepare national reference snake venoms of the species of highest medical impact. Few countries, such as Brazil, have prepared reference venoms (Araújo et al., 2017). In our study, a reference venom of B. asper from Panama has been prepared, including representative venom samples from adult specimens collected in the various regions of this country. This venom has been characterized in terms of its toxicological and enzymatic activities, as well as its electrophoretic pattern in SDS-PAGE. This national reference venom can be used in the preclinical assessment of the neutralizing efficacy of antivenoms distributed in this Central American country. The reference venom from Panamanian B. asper was found to induce lethal, edema-forming, coagulant, hemorrhagic and myotoxic activities. Therefore, this toxicological profile is similar to the one previously described for B. asper venoms from other Central American countries and from Mexico (Gutiérrez, Chaves & Bolaños, 1980; Saravia et al., 2001; Quintero et al., 2007; Segura et al., 2010).
The most important parameter of toxicity of a venom is the Median Lethal Dose (LD50). The Panamanian B. asper reference venom had an LD50 of 91.9 μg (95 confidence limits: 75.6-111.7 μg), which corresponds to a dose of 4.84 mg/kg (95 % confidence limits: 3.98-5.88 mg). This value is similar to the results obtained for adult B. asper venoms from other regions in Latin America, as published by Gutiérrez, Chaves & Bolaños (1980), Otero et al. (1995), Saravia et al. (2001), Gutiérrez, Escalante, & Rucavado (2009) and Segura et al. (2010).
This reference venom from Panama showed high edematigenous and myotoxic activities. Hemorrhagic effect was also observed with this Panamanian reference venom, with a DHm value of 4.93 ± 0.29 μg. The venoms from specific regions of Panama, studied by Martínez (1983b) and Quintero et al. (2007), presented values of 2.5 μg and 6.3 ± 3.6 μg, respectively. It should be taken into account that the reference venom contains snake venoms from all regions of Panama (including the regions studied by these authors), which could explain the intermediate value of the DHm found in this study.
With regards to in vitro coagulant activity, the Panama reference venom had a DCm of 0.536 ± 0.040 μg, which is slightly higher than the reported value for Costa Rican venom (DCm = 0.32 ± 0.02 μg) (Segura et al., 2010) and lower than for Guatemalan venom (3.9 ± 0.08 μg; Saravia et al., 2001). The defibrinating action in vivo of the Panama reference venom was evaluated through the DDm, which was 1.25 μg/mouse. This value is similar to the Colombian venom (DDm = 1.1 ± 0.3 μg) (Otero et al., 1995). However, it appears to be more active than its Costa Rican counterpart (DDm = 3.0 ± 0.5 μg) (Segura et al., 2010).
The proteolytic activity of viperid venoms is associated with coagulopathies, edema, local tissue damage and hemorrhage. The value of the DPm for the Panamanian reference venom was 1.39 ± 0.1 mg. This is similar to those previously reported for the venoms of B. asper of the Atlantic and Pacific regions of Costa Rica, which have values of DPm = 1.2 and 1.4 mg, respectively (Gutiérrez at al., 1985) and slightly higher than that of the B. asper venom from Honduras (DPm = 2.1 mg) (Rojas et al, 1987).
The reference Panama venom showed indirect hemolytic activity (induced by phospholipases A2), with a DHlm of 2.93 ± 0.3 μg. This value is similar to that obtained with B. asper venoms from Antioquia-Chocó (DHlm = 2.0 μg) in Colombia (Otero et al., 1995).
Electrophoretic analysis by SDS-PAGE of the reference venom and of venoms from the different regions of Panama showed qualitatively similar patterns, although quantitative differences were observed, through densitometric analysis, in the percentages of each one of the electrophoretic bands. Previous work analyzed the variation of B. asper venoms from these populations in Panama and showed that, both in their toxic and electrophoretic profiles, these venoms were similar (Velez et al., 2017). However, the small variations found in electrophoretic patterns among the populations highlights the need for including representative samples of snakes from the various regions in Panama, in order to have a truly representative national reference venom preparation.
As for Western blot, the similar patterns of immunorecognition observed between the different antivenoms could be attributed to two aspects: the cross-reactivity and the similarities that exist between the proteins of the venoms of the same genus and species, even if they are from different countries. The high reactivity of the antivenoms of Costa Rica and Colombia may be related to the close similarity in the composition of the B. asper venoms of these countries and their immunological similarities. Both antivenoms include B. asper venom in their antigenic mixture, which explains the high degree of recognition. The experimental monospecific serum obtained from rabbits immunized with Panamanian B. asper venom did not show such strong immunorecognition. This might be due to the fact that commercial antivenoms produced in horses subjected to cycles of repeated immunizations show greater neutralization than experimental antivenoms produced in a single round of immunization (Gutiérrez et al., 2010).
These immunochemical results suggest that the antivenoms tested in this study could be effective in neutralizing the main effects inflicted by the toxins of the venom. However, the preclinical efficacy of antivenoms should be assessed by studying the neutralization of toxic and enzymatic activities of this reference venom, since immunoreactivity by ELISA or Western blot does not necessarily imply neutralization of toxicity (Gutiérrez et al., 2010). For this reason, immunochemical tests, such as Western blotting, should not be used as a basis to recommend the therapeutic use of a specific antivenom (Otero-Patiño, 2009), although they are useful to determine immunological similarities between venoms.
In conclusion, this study described the preparation of a national reference venom of B. asper from Panama, prepared by pooling venom samples from specimens collected in various regions of the country. It is therefore a representative venom from this country. The toxicological, enzymatic, and electrophoretic characterization of this reference venom underscores strong similarities with venoms from this species collected in other countries. In the light of the recommendations of the World Health Organization (WHO), this national reference venom will be useful for assessing the preclinical efficacy of antivenoms distributed in this Central American country.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 

