Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.60 n.1 San José Mar. 2012
Changes in structure and composition of evergreen forests on an altitudinal gradient in the Venezuelan Guayana Shield
*Dirección para correspondencia
Abstract
There have been several ecological studies in forests of the Guayana Shield, but so far none had examined the changes in structure and composition of evergreen forests with altitude. This study describes and analyzes the structure, species composition and soil characteristics of forest stands at different altitudinal zones in Southeastern Venezuelan Guayana, in order to explain the patterns and the main factors that determine the structure and composition of evergreen forests along the altitudinal gradient. Inventories of 3 948 big (>10cm DBH) and 1 328 small (5-10cm DBH) woody stems were carried out in eleven plots, ranging from 0.1 to 1.0ha, along a 188km long transect with elevations between 290 and 1 395masl. It has been found that 1) hemiepihytes become more dominant and lianas reduce their dominance with increasing altitude and 2) the forest structure in the study area is size-dependent. Five families and 12 genera represented only 9% of the total number of families and genera, respectively, recorded troughout the gradient, but the two groups of taxa comprised more than 50% of the Importance Value (the sum of the relative density and the relative dominance) of all measured stems. Moreover, the results suggest that low species richness seems to be associated with the dominance of one or few species. Stand-level wood density (WD) of trees decreased significantly with increasing elevation. WD is an indicator of trees’life history strategy. Its decline suggests a change in the functional composition of the forest with increasing altitude. The Canonical Correspondence Analysis (CCA) indicated a distinction of the studied forests on the basis of their altitudinal levels and geographic location, and revealed different ecological responses by the forests, to environmental variables along the altitudinal gradient. The variation in species composition, in terms of basal area among stands, was controlled primarily by elevation and secondarily by rainfall and soil conditions. There are other interacting factors not considered in this study like disturbance regime, biological interactions, productivity, and dispersal history, which could affect the structure and composition of the forests in the altitudinal gradient. In conclusion, it appears that the structural and floristic variability observed in the studied transect is produced by a combination of different climates and randomly expressed local processes interacting across a complex physical landscape.
There have been several ecological studies in forests of the Guayana Shield, but so far none had examined the changes in structure and composition of evergreen forests with altitude. This study describes and analyzes the structure, species composition and soil characteristics of forest stands at different altitudinal zones in Southeastern Venezuelan Guayana, in order to explain the patterns and the main factors that determine the structure and composition of evergreen forests along the altitudinal gradient. Inventories of 3 948 big (>10cm DBH) and 1 328 small (5-10cm DBH) woody stems were carried out in eleven plots, ranging from 0.1 to 1.0ha, along a 188km long transect with elevations between 290 and 1 395masl. It has been found that 1) hemiepihytes become more dominant and lianas reduce their dominance with increasing altitude and 2) the forest structure in the study area is size-dependent. Five families and 12 genera represented only 9% of the total number of families and genera, respectively, recorded troughout the gradient, but the two groups of taxa comprised more than 50% of the Importance Value (the sum of the relative density and the relative dominance) of all measured stems. Moreover, the results suggest that low species richness seems to be associated with the dominance of one or few species. Stand-level wood density (WD) of trees decreased significantly with increasing elevation. WD is an indicator of trees’life history strategy. Its decline suggests a change in the functional composition of the forest with increasing altitude. The Canonical Correspondence Analysis (CCA) indicated a distinction of the studied forests on the basis of their altitudinal levels and geographic location, and revealed different ecological responses by the forests, to environmental variables along the altitudinal gradient. The variation in species composition, in terms of basal area among stands, was controlled primarily by elevation and secondarily by rainfall and soil conditions. There are other interacting factors not considered in this study like disturbance regime, biological interactions, productivity, and dispersal history, which could affect the structure and composition of the forests in the altitudinal gradient. In conclusion, it appears that the structural and floristic variability observed in the studied transect is produced by a combination of different climates and randomly expressed local processes interacting across a complex physical landscape.
Key words: floristic composition, forest structure, Venezuelan Guayana, altitudinal gradient.
Resumen
A pesar de los diversos estudios ecológicos realizados en los bosques del Escudo de Guayana, ninguno de ellos había analizado hasta ahora los cambios en la composición y estructura de bosques siempreverdes que ocurren al incrementar la altitud. Con el fin de identificar patrones y factores determinantes de la estructura y la composición de bosques en un gradiente altitudinal (290-1 395msnm) en el sudeste de Venezuela se realizaron inventarios de 3 948 fustes grandes (>10cm DAP) y 1 328 pequeños (5-10cm DAP) en once parcelas a lo largo de un transecto de 188km. Con el incremento de la altitud, el área basal aumenta en las hemiepífitas y disminuye en las lianas. Las familias y los géneros más importantes representaron una reducida proporción del total de taxones, pero abarcaron más del 50% del Valor de Importancia. Bajos valores de riqueza se asocian con la dominancia de pocas especies. La densidad de madera decrece significativamente a mayor altitud, y sugiere un cambio en la composición funcional. Existen diferentes respuestas ecológicas de los rodales ante cambios físico-ambientales. La variación de la composición de especies en el gradiente fue controlada por altitud, lluvia y condiciones edáficas; pero se desconoce el efecto de otros factores no considerados como régimen de perturbaciones, interacciones biológicas, productividad e historia de dispersión. La variabilidad observada en la estructura y la composición de bosques en el gradiente parece ser un resultado del efecto combinado de diferentes climas y procesos locales aleatorios que interactúan en un complejo paisaje.
Palabras clave: composición florística, estructura boscosa, Guayana venezolana, gradiente altitudinal.
Forests in tropical regions occur under a wide range of ecological gradients. Tropical forests vary in species composition and structure along altitudinal gradients. Altitude is regarded as an important factor for classifying tropical forests (Beard 1944, Whitmore 1990, Huber 1995).
Different changing site conditions, from the base to the summit of mountains, originates the diversification of habitats. Changes in structure and species composition along elevational gradients were described in the Andes (Grubb et al. 1963, Gentry 1988, Smith & Killeen 1998), Central America (Boyle 1996, Lieberman et al. 1996, Vásquez & Givnish 1998), the Caribbean (Beard 1944, Weaver 2000, Gould et al. 2006), and elsewhere in the tropics (Richards 1976, Whitmore 1990, Mueller- Dombois & Foster 1998). In other neotropical regions, few investigations have studied the changes in composition and structure of forests with altitude (Stadmüller 1987, Gentry 1988, 1995, Clinebell et al. 1995, Kappelle & Brown 2001). This is the case of the forests in the Guayana Shield, which have been little explored (Huber 2006).
The Guayana Shield forests are considered the largest area of undisturbed tropical rainforest that still exists worldwide (Global Forest Watch 2002, Huber & Foster 2003, Hammond 2005). In this region, the most quantitative surveys of forest composition and structure were carried out in lowlands, mainly along rivers and roads, while montane communities (ca. 500-3 000masl) are poorly known (Berry 2002).
Montane forests of the Guayana Shield are located in Venezuela, Brazil and Guyana. Most botanical investigations in this biome are related with general descriptions of plants and with floristic overviews (see Huber 1995). While few quantitative ecological investigations have been published from there (Fanshawe 1952, Veloso et al. 1975, CVG-TECMIN 1987, 1989, Hernández 1994, 1999, Dezzeo & Huber 1995, Dezzeo et al. 1997, Henkel et al. 2002, Henkel 2003, Dezzeo et al. 2004), none has examined the variation of structure and tree composition from lowland to montane forests.
This study compares the characteristics of old-growth forests at different altitudinal levels in the Guayana Shield. The aim is to describe and analyze the structure, floristic composition and soil properties of forests along an altitudinal transect, in order to explain the patterns, and the main controlling factors of floristic composition and structural characteristics of the forests.
Material and methods
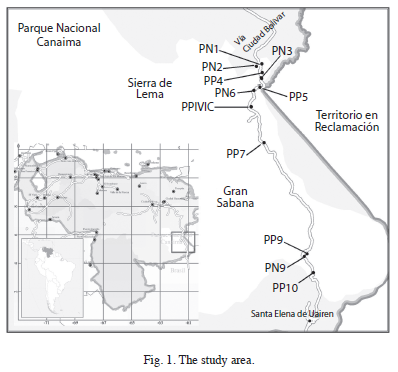
Study area: The study was carried out in the Southeastern Venezuela, along a 188km long transect with elevations between 220 and 1 400masl (Fig. 1). This altitudinal transect runs parallel to the highway from Las Claritas to Brazil, crossing La Escalera (Sierra de Lema) and the Gran Sabana regions, both located in the Canaima National Park.
La Escalera forms part of Sierra de Lema, an irregularly shaped mountain system. Gran Sabana is a high undulating plain with some outstanding table mountains (Tepui) reaching elevations of almost 2 800masl. Both regions are conformed by sedimentary rocks of the Roraima Group of Precambrian age, and some diabase intrusions of the Mesozoic (Huber & Febres 2000, Schubert et al. 1986).
The soils in La Escalera and Gran Sabana are in an advanced phase of weathering. They are characterized by low pH values, deficiency of basic cations, accumulation of acidic cations and low Ca/Al ratio in the soil solution (Dezzeo 1994, Durán 2001, Dezzeo et al. 2004). In some sites the soils have a high skeleton content (35-60%), which reduces the ion exchange and water-holding capacity (Fölster et al. 2001). With the exception of lowland forests, the soils have an organic surface layer with a dense root mat and high humus content. Mineral soil shows a very shallow rooting up to 30cm (Dezzeo 1994, Priess et al. 1999, Dezzeo et al. 2004).
Climate types along transect are distinguished on the basis of the average annual rainfall, average annual air temperature and altitudinal level. Below 500-600masl, a macrothermic humid climate with basimontane forests (>2 000mm rainfall and >24ºC) predominates, with either a short or no dry season at all. In the uplands between 500 and 1 200-1 400masl, rainfall increases and temperature decreases (0.6ºC per 100m altitude) and submontane forests dominate in a submesothermic perhumid climate (>2 000mm and 18-24ºC). Above 1 400masl, a mesothermic perhumid climate with montane forests (>2000mm and 12-18ºC) prevails (modified after Huber 1995). Regarding the distribution of rainfall throughout the year, weather stations at Km 88 (macrothermic climate) and at Sierra de Lema (mesothermic climate) did not register a monthy average with less than 100mm of precipitation (Ortíz et al. 2006). At the Station of Yuruaní (submesothermic climate), Hernández (1994) reported an average minimum of 50mm rainfall in February. Nevertheless, the high amount of annual rainfall masks the possibility of seasonal water stress in shallow rooted forest soils (Dezzeo 1994). Priess (1996) estimated in a Gran Sabana´s forest on stony soils, a range of 59-66days during 1990-1991 without plant available water.
Although several regional floristic studies have been carried out (Steyermark 1966, Hernández 1999, Durán 2001, Dezzeo et al. 2004), the botanical knowledge is still fragmentary. New plant species are frequently reported for this region (Berry et al. 1995, Sanoja 2004, 2009b), and plant endemism is high. Picón (1995) reported 310 endemic species of vascular plants for the Gran Sabana and surroundings. Nearly 13% of the tree species in the cloud forests of Sierra de Lema are endemic (Sanoja 2009).
Sierra de Lema is covered by different evergreen forest types (Huber 1995). In the Gran Sabana, forests are considered as the potential natural vegetation, however the current vegetation is dominated by savanna with scattered forest patches, disturbed by frequent fires.
Five bioclimatic evergreen forests can be distinguished along the altitudinal transect (hereafter referred to as forest sections). The three forest sections in the North belong to a sequence of large forests ascending Sierra de Lema: basimontane forest (BMF), lower-montane forest (LWF) and cloud-montane forest (CMF). The remaining two forest sections are Gran Sabana´s fragments of montane forests (FMF) in the North and lower-montane forests (FLMF) in the South.
Forest inventory plots: Forest inventory plots of this study are the same permanent plots established by Universidad Nacional Experimental de Guayana (UNEG) and Electrificación del Caroní (EDELCA) for a broader long-term ecological research on regional forests dynamics (Hernández & Castellanos 2006). The plots were selected using field reconnaissance and visual interpretation of radar images, aerial photographs and LANDSAT images. Selection criteria were accessibility, topographical homogeneity, altitudinal level, well drained soils and apparently non-disturbed old-growth forests with canopy dominated by non-pioneer species. A total of 11 plots were established along an altitudinal transect of 188km, by placing two or three plots in each of the five forest sections. Plot sizes range from 0.1 to 1.0ha, Fig. 1 and Table 1 show their location and characteristics.
Soil sampling procedures: In each forest plot, organic surface layer and mineral soil were randomly sampled at six points using an AMS regular soil auger. Each soil sample was packed in one liter bag, filled to about twothirds (approximately 400g). Mineral soil was collected at 0-20 and 20-40cm depths. Some samples were collected only up to 20cm, when the C-horizon was reached. Each collected organic sample was dried, milled and digested with a mixture of H2SO4-H2O2 (according to Tiessen & Moir 1993). The extracts were colorimetrically analyzed for total N and P with the Technicon Auto-Analyzer. Ca, K and Mg were determined in the same extract by atomic-absorption spectrophotometry. Total C was determined through complete combustion of the samples at 500°C for eight hours in amuffle furnace (Davies 1974)
Mineral soil samples were dried and passed through a 2mm soil sieve. Soil texture was determined by the hydrometer method (Day 1965). Soil pH was measured in 1M KCl (Thomas 1982). Organic matter was determined by the Walkley & Black (1934) method. Total N was measured following the Kjeldahl method (Jackson 1976). Available P was extracted according to Tiessen & Moir (1993), and colorimetrically determined (Murphy & Riley 1962). Exchangeable K, Mg and Ca were determined by spectrophotometry of atomicabsorption using 1N ammonium acetate as extracting solution (Thomas 1982). Exchangeable Al was extracted using a 1M KCl solution and determined by titration using NaOH (McLean 1965).
Vegetation recording: Each delimited plot was divided into 0.1ha-subplots. In each subplot, all stems greater than 10cm diameter at breast height (DBH at 1.3m height) were tagged, mapped and identified by their local indigenous name (Pemón-Arekuna). Their DBH, total height and life form (trees, hemiepihytes and liana) were assessed. In each plot, stems between 5-10cm DBH were measured in one subplot of 0.1ha.
Stems were assigned to morphospecies, whenever possible, either in the field or by collecting voucher specimens for posterior comparison with herbarium samples. In general, identification to the species-level was difficult because most of collected botanical samples were sterile. About 5 000 botanical samples were collected and deposited at Herbario Nacional de Venezuela (VEN) and Herbario Regional de Guayana (GUYN) for identification and cross-referencing. The nomenclature follows Berry et al. (1995).
The vegetation data were analyzed by forest section, plot and subplot. In plots with more than one subplot, the results were presented as mean values with their respective standard deviation. For each plot stem density, basal area, number of tree species (excluding lianas) and Importance Value (“IV”, defined as the sum of the relative density and the relative dominance) by each family and species were calculated. Additionally, diversity was estimated using α Fisher’s Alpha index (Magurran 1988).
Wood specific gravity or wood density (WD) value was assigned to every measured stem. WD data for tree taxa were compiled from different sources (Brown 1997, Fearnside 1997, Worbes 1999, Aguilar-Rodríguez et al. 2001, ter Steege & Hammond 2001, Schoengart 2003, Baker et al. 2004, Chave et al. 2006, Méndez 2006, Ortíz et al. 2006, Mújica 2007, Zanne et al. 2009). At species level, WD could be determined for 25% of all stems. WD is closely dependent on phylogeny. The differences between the genera account for the largest proportion of the total variation (Baker et al. 2004). Therefore, in the absence of species-level data for individual stems, WD values were allocated generic-level (30% of stems) or family-level (28%) mean values. The remaining 17% of stems corresponded to non-identified stems or taxa without available information. For these, the neotropic overall species-level mean (0.65g/cm3), was used as calculated by Chave et al. (2006).
For data analysis: The variation of basal area and average wood density were compared between study plots using analysis of variance (one-way ANOVA) with Minitab version 13 (Meyer & Krueger 2004) to test for significant differences in altitudinal variation of floristic composition and structure.
A nonlinear regression analysis (NLREG) revealed the relationship between plots in terms of basal area of morphospecies. A Durbin- Watson test was carried out to avoid misuse of such analysis (Legendre et al. 2005), and to detect the presence of autocorrelation among study plots in the residuals from the regression analysis, using elevation as independent variable and basal area as dependent variable. It was assumed that if the percentage variation is well represented, any other factor would not contribute to the variation. Otherwise, if the variation could not be explained, we proceeded to test species composition by environmental variables. A Canonical Correspondence Analysis (CCA) was applied according to Legendre et al. (2005), analyzing the variation of basal area of morphospecies versus elevation, precipitation and soil variables, in which number of dry months per year and soil texture were excluded because of their high variance inflation factor. CCA is the ordination method most frequently used in vegetation science (Wehrden et al. 2009). It enables an evaluation of the influence of the environment on the composition and structure of forests, by providing estimates of the contributions of the response and explanatory variables to the canonical relationships, as well as the visual analysis of patterns in ordination biplots (Legendre et al. 2005).
In relation to the soil data, mean values and standard deviations were provided throughout the presentation of similarities/differences for the texture and chemical properties of the soils among forest sections.
Results
Soil properties: All studied forest sections presented similar soil texture, with high sand content and relatively low clay content. An exception was FLMF, which presented lower sand and higher clay contents (Table 2). The mineral soil of all forest sections was covered by a relatively thick organic surface layer (5-7cm deep in average), which was characterized by a network of fine roots mixed with litter in various stages of decomposition. BMF was an exception presenting a thin organic layer (0.8-0.9cm deep). The concentrations of C and nutrients (N, Ca, K and Mg) in the organic surface layer were 2-50 times higher than in mineral soil (Table 2). In general, the mineral soil was characterized by low pH values, high organic matter concentration, low concentration of nutrients (N, P, Ca, K and Mg) and high concentration of Al, reflecting a high degree of weathering of the parent material (Table 2). Although all soils studied were strongly acidified and poor in nutrients, some differences were found in the element concentrations among the selected forest sections. These differences, however, seem not to be related to the altitudinal position. CMF soil showed 1.4 to 4.5 times more Ca and 1.8 to 3.8 times less N than the soils of the other forest sections. The K levels in the soils of FLMF were 1.5 to 5 times higher than in the other studied soils, while the highest concentrations of Mg were found in the soils of CMF and LMF. The Al concentrations were 1.4 to 3.0 times higher in the soils of the FMF than in the other forest sections (Table 2).
Forest community structure: Structural characteristics of the altitudinal forest sections are presented in Table 3. Stem density over the gradient ranged between 65 and 101 stems (≥10cm DBH) per 0.1ha, and tended to increase slightly with altitude. The highest mean stem density was achieved at FMF in conjunction with the lowest average diameter.
High levels of basal area (≥3.7m2/0,1ha) were combined with high stem densities (≥100 stem/0.1ha) or high average diameter (≥22cm, Table 3). The coefficient of determination in the quadratic regression analysis among basal area and elevation indicated a low variation percent; only 23% of the variation in basal area is accounted for the elevation variable (Y=11.09-2.52X+0.21X2). Despite this low percentage, the associated ANOVA showed significant differences (DF=8, F=7.82, p<0.02).
The basal area of small (5-10cm DBH) and medium (10-30cm DBH) trees tended to rise with higher altitude. A difference between Sierra de Lema and Gran Sabana was that the highest basal area in the medium trees occurred in the Gran Sabana (Table 4a). However, the most important difference was that large trees with diameter ≥70cm DBH have been recorded only in the forests of Sierra de Lema, but not in the forest fragments of the Gran Sabana. A higher proportion of large trees in terms of basal area were found at BMF and CMF. Despite the low proportion of large trees (0.3-3% of the total density), they can reach a significant proportion of the stand’s basal area (up to 24-31%) (Table 4b). Most frequent large individuals belonged to Mora gongrijpii, Couma rigida, Tachigali sp., Sextonia rubra, Micropholis spectabilis, Vochysia costata, Eschweilera sp. and Elvasia brevipedicellata.
Average wood density (WD) at stand level decreased with increasing elevation (Table 3), underlined by the significant difference of WD between plots (df=10, F=136.44, p<0.0001). The values of stand’s average WD at BMF were higher than in the rest of forest sections, and than the mean of the neotropic species (0.65g/cm3).
Physiognomy and woody life forms: The highest proportion (16.2%) of basal area of well-marked buttressed trees was reached at BMF. In the other higher altitudinal forest sections buttresses were smaller (2.5% at LMF, 1.8% at FMF) or absent (CMF, FLMF). Most frequent buttressed trees belonged to Mora gongrijpii, Pouteria sp. and Eschweilera sp.
The proportion of big lianas (≥10cm DBH) of total basal area was comparable among BMF (0.5%), LMF (0.6%) and FLMF (0.4%), whereas at CMF and FMF the proportion was smaller (0.3% and 0%, respectively). Most abundant liana genera were Bauhinia, Dalbergia and Davilla.
Hemiepiphytes’ (“strangling” trees) proportion of total basal area was 0.1% at BMF, 1.7% at CMF, 0.2% at FLMF and 0% at the other altitudinal forest sections. Hemiepiphytes taxa were Clusia spp., Coussapoa spp., Ficus spp., Oreopanax capitata and Schefflera quinquestylorum.
Richness: A total of 3 948 big (>10cm DBH) and 1 328 small (5-10cm DBH) woody stems corresponding to 220 indigenous names were recorded in all plots. They belong to 385 woody morphospecies in 141 genera and 58 families. Given the difficulties with the identification process, the exact number of species still remains uncertain, so the estimates are preliminary. About 53% of the morphospecies were identified to species-level, 35% to genus level and 12% to family-level. Six woody taxa with sterile vouchers could not be identified at any level.
Species-and genera richness varied among plots and differed by almost three-fold and two-fold, respectively (Table 5). PP4 in LMF was the stand with the highest values of species- richness and diversity indices (α Fisher’s).
Considering the great variation in the number of stems among plots, the accumulated species number was estimated as a function of the quantity of individuals (species number per 60 stems). This approach showed similar patterns as before, where the species richness among plots differed by three-fold (Table 5) and the greatest species-richness was reached again in the same LMF plot.
Composition: Families of trees with higher overall species richness on the entire gradient were Leguminosae sensu latu (33 species), Lauraceae (22), Sapotaceae (21), Rubiaceae (18), Burseraceae (16), Chrysobalanaceae (15) and Clusiaceae (12).
The predominant families of trees with respect to their Importance Values were Leguminosae, Lauraceae, Burseraceae, Sapotaceae, Chrysobalanaceae, Arecaceae, Lecythidaeae, Clusiaceae, Rubiaceae and Annonaceae (Table 6a). 12 genera (Dimorphandra, Alexa, Clathrotropis, Emmotum, Eperua, Eschweilera, Licania, Micropholis, Mora, Protium, Sextonia, Tachigali, data not shown) and five families (Caesalpinaceae, Fabaceae, Lauraceae, Sapotaceae, Burseraceae, Table 6a) make up 50% of the IV of all measured trees in the gradient, but they represented only 9% of the total number of genera and families recorded in this study. The only family that appeared within the group of the five most important families in all altitudinal forest sections was Leguminosae (Table 6b). This family was the most dominant family at BMF and FMF, whereas Lauraceae was the most dominant at CMF.
Species composition varied continuously among the altitudinal forest sections (Table 7).
Variation of structure and composition with respect to environmental components: The Durbin-Watson test indicated that basal area among study plots was not auto-correlated through their geographic proximity and spatial separation. Thus, plots were independent and related to altitude. Regression analysis found a nonlinear relationship between basal area and elevation along the gradient plots, describing it as a quadratic function. To explain this function a Principal Component Analysis (PCA) was performed first, which was applied to reduce the full set of 11 environmental variables to those with lower variance inflation factor (VIF), avoiding multicollinearity of highly correlated variables (ter Braak 1986). Only the environmental variables with lower VIF, namely elevation, precipitation, organic matter (OM%), N%, pH, P and silt% (data not shown), were considered in CCA.
CCA showed differential ecological responses of the plots along different gradients of environmental variables (Table 8), indicating that the basal area is restricted to 21.8% of the variance explained by the linear combination of these environmental variables. The first ordination axis was extracted by the elevation gradient with an eigenvalue (λ) of 0.96, where the CMF plots PP5 and PN6 are distributed along the elevation gradient. The precipitation gradient (λ=-0.91) was extracted by the second axis (Table 8). Then the remaining five axes were extracted by soil properties (N%, P and OM%). The first three axes of CCA account for 56.8% of the variance in the basal area data set, showing a clear correlation with the environmental variables of elevation and rainfall.
Visually, the ordination bi plot (Fig. 2) showed a pattern where the CFM stands are located on the far right side and the BMF stands on the leftmost side. The difference in elevation between these two extremes corresponds to the maximum altitudinal range among the study plots. The remaining plots, that belong to the mid-elevation, were arranged in intermediate positions of the ordination gradient (Fig. 2), between the two altitudinal extremes. Thus, CCA results produced an ordination of stands placed by geographic position and altitudinal level, which is consistent with the previous differentiation into the five altitudinal forest sections.
Discussion
Forest Patterns in composition and structure: The floristic and structural distinction of the forests on the basis of their altitudinal levels and geographic location indicated a high spatial and biotic heterogeneity of the forests along the study transect. The identified patterns of (1) life forms, (2) structure, (3) floristic composition and (4) functional composition are discussed below.
(1) Life forms: High basal area of big lianas were recorded in basimontane and lower montane forests, while the largest basal area of strangling trees (hemiepipyhites) were found in cloud forests, suggesting that with increasing altitude hemiepipyhites become more dominant and lianas less. A possible explanation of the increase of hemiepipyhites in cloud forests may be the more humid climate, because water stress could limit hemiepipyhites development (Weiblen 2002).
(2) Structure: Several characteristics indicated that the forest structure along the altitudinal gradient is size-dependent. For example, stem density increased slightly with altitude, showing the largest density value in conjunction with the lowest average diameter. High levels of basal area were presented in combination with high values of stem density or average diameter. Despite the large number of stems in the small (5-10cm) and medium (10-30cm) DBH classes, most of the contribution to the total basal area in all forest stands is derived from trees bigger than ≥30cm. While basal area of the small and medium trees tended to rise with altitude, the large trees (>70cm) were found only in continuous forests of Sierra de Lema at all altitudinal levels, but not in the forest patches at any level in the Gran Sabana. Due to their large sizes, the large trees accounted for a large proportion of basal area despite their low numerical abundance.
This apparent size-dependence suggests that trees in montane forests tend to be more numerous but smaller. Therefore, potential spatial scale of competition for space may tend to be smaller in montane forests. Stem densities in our lowland (BMF) plots fell into the range of 29-170 stems per 0.1ha (≥10cm DBH) registered for different tropical lowland forests (30-760masl) (Grubb et al. 1963, Condit et al. 1996, Oliveira et al. 2008). Stem-densities in the remaining forest sections (LWF, FLMF, CMF and FMF) were slightly larger than the range (23-85 stems per 0.1ha), documented (Grubb et al. 1963, Boyle 1996) for several montane tropical forests (700-2 830masl), possibly due to a stronger disturbance regime, as discussed below.
(3) Floristic composition: Floristic composition varied continuously along the altitudinal transect, often with a few taxa comprising most of the community dominance. All important families in this study belong to the 16 most important families of trees in lowland forests of Amazonia and Guayana Shield (ter Steege et al. 2000). An exception was Clusiaceae as the only dominant family well represented in other premontane forests of the Neotropics (Gentry 1995). Leguminosae was always in the group of the most important families in all altitudinal levels. Leguminosae is widespread in the neotropical forests (Gentry 1995, 1988), and predominant in Eastern Amazonia and in the Guayana Shield (ter Steege et al. 2000, Berry 2002), but rare in the biogeographic province of Pantepui above 1 500masl (Riina 1996). Leguminosae predominance in the Guianas has been associated with a better adaptation of some species to prevailing low-fertility soil conditions (ter Steege & Hammond 1996, Henkel et al. 2002).
Some of the predominant species like Caraipa tereticaulis, Protium spp., Licania spp., Dimorphandra macrostachya, Clathrotropis macrocarpa, Eschweilera.spp. and Alexa spp. has been reported as dominant taxa in other forests in the Venezuelan Guayana (Steyermark & Nilsson 1962, Steyermark 1966, Veillon 1985, Huber 1995, Hernández 1999) and Guyana (ter Steege 2000, ter Steege et al. 2006). Licania and Protium are among the top ten genera with the greatest abundance of trees in the Amazon (ter Steege et al. 2006).
Due to the low collection density of herbarium specimens in the Guayana Shield, the current geographical distribution of many taxa is hardly known (Berry 2002). As usual in small tropical forest plots, most of the species sampled were represented by one or two individuals on a single site (Gentry 1988). Thus, they were not adequately sampled to draw any conclusions about habitat specificity. Altitudinal distribution of each species in studied samples was variable. Some species are distributed over a restricted altitudinal range like Mora gonggrijpii and Alexa cowanii, recorded only at BMF, or Podocarpus tepuiensis and most of the endemics registered only in montane forests. Other species showed wider altitudinal ranges including Annona symphyocarpa, Parkia nitida, Symphonia globulifera and Simarouba amara. There was some overlap in terms of the most important genera and species. Likewise, the forest stands closer to each other shared several common species, which suggests that changes in species assemblage could be gradual.
The broad variation in species richness showed a peak at intermediate altitudinal levels, and a decrease in the PPIVIC montane forest fragment. The lowest diversity in PPIVIC could be attributed mainly to the high degree of stand dominance by Dimorphandra macrostachya. The analysis of the species number, density and basal area of the more important species suggest that low richness seems to be associated with dominance of one or few species (Condit et al. 2005). D. macrostachya, Mora gonggrijpii and Sextonia rubra, covered about 30% of all trees or total basal area in FMF, BMF and CMF, showing a stronger dominance. Contrarily, the important species in the remaining forest sections (LMF and FLMF) made up only 6 to 7% of all trees or total basal area, showing less dominance, which was expected in the case of LMF stands due to their higher diversity. Environmental stability and low disturbance regimes leading to competitive exclusion have been used as arguments to explain similar patterns of local tree dominance in lowland forests in the Guayana Shield (Davis 1941, Hammond & Brown 1995, ter Steege & Hammond 2001).
(4) Functional composition: The wood density (WD) of trees decreased significantly with increasing elevation. According to the classification of ter Steege (2003) the average WD of the trees in BMF and FLMF correspond to the hardwoods, whereas trees in LMF, CMF and FMF correspond to the softwoods. The shift of species composition along the study transect caused the regional gradient in WD. Given such changes in taxonomic composition and in WD, it may be assumed that a change also occurs in the functional composition with increasing altitude. WD varies widely between tropical forest tree species and sites, and is a convenient indicator of the life history strategy of trees, because it is closely related to differences in diameter growth rates and in life history strategies like light demand. Slowgrowing shade tolerant species typically have higher WD than fast-growing light demanding species (Whitmore 1989).
The most important species of stands at high altitude showed a lower WD than that ofthe important species of stands at lower altitudes, repeating the same pattern observed at community-level. D. macrostachya, S. rubra and M. gonggrijpii with the highest proportion of IV in the gradient, reach the tree categories of canopy and emergent. The first species dominated in FMF and is softwood (0.45g/cm3), and the second prevailing in CMF a medium hardwood (0.53g/cm3). Contrarily, M. gonggrijpii a hardwood tree (1.03g/cm3) dominated in BMF. It has been assessed to have an annual diameter increment between 0.38cm/yr for M. gonggrijpii and 0.45cm/yr for D. macrostachya (Hernández & Castellanos 2006). Such growth rates and WD support the idea of differences in life history strategies among these species. However, the slower annual diameter increment for S. rubra of 0.33cm/yr (idem) does not. This species reached diameters up to 110cm and heights up to 38m. Due to the lack of information the functional group of S. rubra could not be established.
Trees of D. macrostachya reached diameters up to 70cm and heights up to 35m. Despite its relative medium seed-size, the trees produce numerous seeds (until 12-34% of the total seed pool) in mature and secondary forests (Flores & Dezzeo 2005). D. macrostachya seedlings in the study region presented high abundance (30- 39% of all seedlings), high survival rates (48%) and high mortality (50%) (Dezzeo et al. 2008, Rettenmaier & Fölster 1999). This species has been recorded in all diameter tree classes, and showed high abundance in secondary forest regeneration (Hernández 1999, 1994). D. macrostachya could be regarded as an opportunist canopy tree species, and it seems to be a long-lived species which possibly grows up following destructive events. Given fortuitous regeneration following major disturbance, D. macrostachya could become established and dominate the canopy of forest for a long time. Dominance of D. macrostachya could be promoted by the combination of the prevailing poor soils conditions and the occasional larger disturbances of severe droughts, extensive fires and possibly large forest blow-downs produced by occasional heavy windstorms (Nelson et al. 1994, Terborgh & Foster 1998), specially at higher altitudes in the study region, where wind speed tends to increase (Esteban Perdomo pers. com.). In these situations a top competitor particularly tolerant to disturbance stressors such as fire or drought may be dominant (Hart 1990).
M. gonggrijpii, has been recorded only at BMF, where its emergent and canopy trees reached diameters over 100cm and heights up to 45m. M. gonggrijpii has been characterized as a large seeded (climax) species (ter Steege 1994). Mora species are thought to be strongly shade tolerant in their seedling stage, showing abundant regeneration and recruitment (Bell 1969, Richards 1976, ter Steege et al. 1993), and producing a large seed crop (ter Steege 1994). M. gonggrijpii is common on non-flooded substrate (ter Steege 1990), and could become dominant (Rollet 1969, González 2006), or even mono-dominant (ter Steege 2000).
Factors controlling structure and composition of forests: this study revealed the different ecological responses of the forest stands along the gradient to the environmental variables. According to the CCA, the variation in species composition in terms of basal area among stands (beta diversity) was controlled primarily by (i) elevation and secondarily by (ii) rainfall and (iii) soil conditions.
(i) Elevation: Forest structure and species composition varied broadly among altitudinal levels and regions and to a lesser degree within each altitudinal level. Since basal area among study plots is not auto-correlated through their geographic proximity and spatial separation, plots are independent and associated with altitude. According to the regression analysis and the associated ANOVA, the altitude explained 23% of the variation in basal area in the gradient. The explanation for the remaining 77% must be sought in other interacting factors (ter Steege 2006).
(ii) Rainfall: the secondary role of precipitation was expected, because of the annual and monthly rainfall changes along the studied transect. Despite absence of water deficit under the average climate, occasional years with severe droughts have been reported (Koch-Grunberg 1917, D. Holdridge cited by Tate 1932, Röhl 1948, Vila 1975, Williams et al. 2005). During these drought periods extensive forest fires have occurred (D. Holdridge cited by Tate 1932, Röhl 1948, Bernardi 1957). Palynological- and 14C analysis also showed the occurrence of fire events and vegetation changes in the Gran Sabana since the Early Holocene (Rull 1991, 1992, 1999, 2009, Fölster 1992, Schubert et al. 1986).
(iii) Soil conditions: the role of soil as controlling factor at the lower elevations could possibly be more important than at higher elevations. This is suggested by the low fertility of mineral soil in all plots and the increasing importance of the organic surface layer as nutrient supplier and buffer factor on the sites at the higher altitudes, where thick organic surface layers with a dense network of roots were prevailing, in contrast to the thin layers in lowland forests. According to Fölster et al. (2001) and Dezzeo et al. (2004), the organic surface layer on the soils of the Gran Sabana´s forests plays an important role to maintain soil fertility. Their absence could be a critical factor determining the capacity of the forests to recover from drought and fire disturbances.
The results of this study indicate the dominance of environmental control as drivers of species assemblage along the altitudinal gradient and partially support the hypothesis that species distribution is related to environmental conditions (Gentry 1988, Tuomisto et al. 1995). Nevertheless, other interacting factors not considered here could affect the structure and composition of the forests along the altitudinal gradient. These factors may be disturbance regime, biological interactions (Pitman et al. 1999, 2001), productivity and dispersal history (Hubbell 2001). Unfortunately such information is not available for the study region and therefore the relative influence of these factors in shaping the current spatial patterns of structure and composition of the forests is unknown. However, some deductions are possible, for example, the decline in floristic similarity with distance between stands suggests a role for dispersal limitation, although this could have been masked by the difference in climate along the gradient.
WD can be used as an indicator of historical dynamics of community disturbance, since the distribution of tree life history strategies, and therefore of WD within a forest, reflects the community responses to disturbance, successional patterns and spatial patterns. It is also expected that the distribution of WD within a tropical forest varies among sites depending on the disturbance regime and the soil fertility (Muller-Landau, 2004, ter Steege & Hammond 1996). WD, in several neotropical forests, has shown significant decreases with increasing altitude (this study, Chave et al. 2006, Swenson & Enquist 2007), as well as demonstrating a close relationship with levels of disturbance (ter Steege & Hammond 1996) and soil fertility (Baker et al. 2004, Muller-Landau 2004). The spatial shifting pattern of composition of species and of functional guilds found along the studied transect, represents a gradient from lowland to montane forest sections, each one dominated by certain types of species. Considering the prevailing infertile edaphic conditions, it is plausible that the dynamics of the lowland forests can be different from the dynamics of the montane forests in Sierra de Lema and Gran Sabana, the latter two could also differ among themselves.
The results of higher WD at stand level in the BMF agree with the notion proposed by ter Steege & Hammond (2001) that in lowland forests of the Guayana Shield an inherently low-intensity disturbance regime prevails. Our basimontane forests could have such lowintensity disturbance regime, but this could not be valid for the lower-montane and montane forests on the altitudinal gradient, which showed low average stand WD and therefore we would rather expect a high-intensity disturbance regime. Such expectation is supported by the gap sizes of a cloud forest in the Sierra de Lema, which were higher than those reported for most tropical forests (Durán et al. 2011).
Another possible indicator of a high disturbance level could be the notable lack of large stems (≥70cm DBH) in the patches of lower-montane and montane forests in the Gran Sabana. Such lack could have been promoted by progressive fragmentation, lower availability of water through more seasonal precipitation in comparison with La Escalera and more frequent and intense perturbations. Laurance et al. (1998, 2000) proposed that progressive fragmentation and advancing changes of edgeeffects accelerate turnover by killing large trees and replacing them by much smaller trees, leading to a marked loss of aboveground tree biomass in Central Amazonas. Forest patches are more prone to repeated disturbances of wind and fire, which can kill and damage many trees (Laurance et al. 1997, Nepstad et al. 2007, Barlow & Peres 2008). Additional support to the idea of such high disturbance level is the difficulty of occurrence of low rates of disturbance, when soil conditions create a constant chemical stress that makes the forest vegetation extremely sensitive to drought and fires (Fölster et al. 2001).
In conclusion, it appears that the structural and floristic (beta diversity) variability observed in the studied transect are produced by a combination of different climates (altitude and precipitation) and randomly expressed local processes (e.g. disturbance events and recruitment limitation) interacting across a complex physical landscape (substrate).
Acknowledgments
We thank Mayerslin Echagarai, Haiddye Durán, Litzinia Aguirre & Jesús Salazar for their field work, Albert Reif & James Ross- Jones for their input on the manuscript and Juan López for preparing the map. Funding and logistical support provided by UNEG, CVG-EDELCA & IVIC. PDV & INPARQUES provided logistical support.
Dedicated to Horst Fölster† to honor his contribution to forest ecology in the region.
References
Aguilar-Rodríguez, S., L. Abundiz-Bonilla & J. Barajas-Morales. 2001. Comparación de la gravedad específica y características anatómicas de la madera de dos comunidades vegetales en México. Anales del Instituto de Biología UNAM 72: 171-185. [ Links ]
Baker, T., O. Phillips, Y. Malhi, S. Almeida, L. Arroyo, A. Di Fiore, T. Erwin, T. Killeen, S. Laurance, W. Laurance, S. Lewis, J. Lloyd, A. Monteagudo, D. Neill, S. Patiño, N. Pitman, J.N. Silva & R. Vásquez Martínez. 2004. Variation in wood density determines spatial patterns in Amazonian forest biomass. Global Change Biol. 10: 545-562. [ Links ]
Barlow, J. & C. Peres. 2008. Fire-mediated dieback and compositional cascade in an Amazonian forest. Philos. Trans. R. Soc. London Ser. B 363: 1787-1794. [ Links ]
Beard, J.S. 1944. Climax Vegetation in Tropical America. Ecology 25: 127-158. [ Links ]
Bell, T.I.W. 1969. An investigation into some aspects of management of the Mora excelsa Benth forests of Trinidad with special reference to the Matura Forest Reserve. Ph.D. Thesis, University of the West Indies, Port Spain, Trinidad and Tobago. [ Links ]
Bernardi, A.L. 1957. Estudio botánico-forestal de las Selvas Pluviales del Río Apacará, región de Urimán, estado Bolívar. Publ. Dir. Cult. Univ. de los Andes 63: 150. [ Links ]
Berry, P. 2002. Floristics of the Guayana Shield. Working paper for the floristic group during the Guayana Shield Conservation Prority Setting Workshop. Conservation International. UNDP. UICN-Netherlands, Paramaribo, Surinam. [ Links ]
Berry, P., O. Huber & B. Holst. 1995. Floristic analysis and phytogeography, p. 161-192. In P. Berry, B. Holst & K. Yatskievych (eds.). Flora of the Venezuelan Guayana. Vol. I: Introduction. Missouri Botanical Garden, St. Louis and Timber, Portland, Oregon. USA. [ Links ]
Boyle, B.L. 1996. Changes on Altitudinal and Latitudinal Gradients in Neotropical Montane Forests. Ph.D. Thesis, Washington University, St. Louis, USA. [ Links ]
Brown, S. 1997. Estimating biomass and biomass change of tropical forest. FAO Forestry paper, 134. FAO, Rome, Italy. [ Links ]
Chave, J., H. Muller-Landau, T. Baker, T. Easdale, H. ter Steege & C. Web. 2006. Regional and phylogenetic variation of wood density across 2456 neotropical tree species. Ecol. Appl. 16: 2356-2367. [ Links ]
Clinebell, R., O. Phillips, A. Gentry, N. Stark & H. Zuuring. 1995 Prediction of neotropical tree and liana species richness from soil and climatic data. Biodiversity Conserv. 4: 56-90. [ Links ]
Condit, R., P. Ashton, H. Balslev, N. Brokaw, S. Bunyavejchewin, G. Chuyong, Co. L, H. Shivaramaiah, S. Davies, S. Esufali, C. Ewango, R. Foster, N. Gunatilleke, S. Gunatilleke, C. Hernández, S. Hubbell, R. John, D. Kenfack, S. Kiratiprayoon, T. Hart, A. Itoh, J. Lafrankie, I. Liengola, D. Lagunzad, S. Loo de Lao, E. Losos, E. Magard, J. Makana, N. Manokaran, H. Navarrete, S.M. Nur, T. Okhubo, R. Pérez, C. Samper, L.H. Seng, R. Sukumar, J.C. Svenning, S. Tan, D. Thomas, J. Thompson, M.I. Vallejo, G. Villa-Muñoz, R. Valencia, T. Yamakura & J.K. Zimmerman. 2005. Tropical tree diversity: results from a network of large plots. Biologiske Skrifter 55: 565-582. [ Links ]
Condit, R., S. Hubbell, J.V. Lafrankie, R. Sukumar, N. Manokaran, R. Foster & P.S. Ashton. 1996. Speciesarea and species –individual relationships for tropical trees-a comparison of three 50 ha plots. J. Ecol. 84: 549-562. [ Links ]
CVG-TECMIN. 1987. Proyecto Inventario de los Recursos Naturales de la Región Guayana. Hojas NB-20-4, NB-20-8, NB-20-12, NB-20-16, 8 volumes, maps 1:500,000. Ciudad Bolívar. Venezuela. CVG-TECMIN. [ Links ]
CVG-TECMIN. 1989. Proyecto Inventario de los Recursos Naturales de la Región Guayana. Hojas NB-20-3, NB-20-7, NB-20-11, NB-20-15, NA-20-3, 8 volumes, maps 1:500,000. Ciudad Bolívar. Venezuela. CVG-TECMIN. [ Links ]
Davies, B.E. 1974. Loss on ignition as an estimate of soil organic mater. Soil Sci. Soc. Am. Proc. 38: 150-151. [ Links ]
Davis, T.A.W. 1941.On the island origin of the endemic trees of the British Guiana peneplain. J. Ecol. 29: 1-13. [ Links ]
Day, P.R. 1965. Particle fractionation and particle size analysis, 985-994. In C.A. Black (ed.). Methods of Soil Analysis. Part 1. Agronomy. [ Links ]
Dezzeo, N. (ed.). 1994. Ecología de la Altiplanicie de la Gran Sabana I. Scientia Guaianae 4. Caracas. Venezuela. [ Links ]
Dezzeo, N. & O. Huber. 1995. Tipos de bosque sobre el Cerro Duida, Guayana Venezolana, p. 149-158. In S.P. Churchill, H. Balslev, E. Forero, J.L. Luteyn (eds.). Biodiversity and Conservation of Neotropical montane forests. The New York Botanical Garden, New York, USA. [ Links ]
Dezzeo, N., L. Hernández & H. Fölster. 1997. Canopy dieback in humid submontane forests of Alto Uriman, Venezuelan Guayana. Plant Ecol. 132: 197-209. [ Links ]
Dezzeo, N., N. Chacón, E. Sanoja & G. Picón. 2004. Changes in soil properties and vegetation characteristics along a forest-savanna gradient in Southern Venezuela. For. Ecol. Manage. 200: 183-193. [ Links ]
Dezzeo, N., S. Flores & N. Chacón. 2008. Seedlings dynamics in undisturbed and adjacent disturbed forest in the Gran Sabana, Southern Venezuela. Interciencia 33: 273-279. [ Links ]
Durán, C. 2001. Estructura y composición florística de los bosques de Sierra de lema, con especial énfasis en Pourouma bolivarensis C.C. Berg. Informe de pasantía. Facultad de Ciencias Forestales y Ambientales, Universidad de los Andes, Mérida, Venezuela. [ Links ]
Durán, C., A. Reif ; L. Hernández. 2011. Understanding small scale disturbances in the Guayana´s Montane Forests: Gap characterization in Sierra de Lema, Venezuela. Interciencia 36: 272-280. [ Links ]
Fanshawe, D.B. 1952. The vegetation of British Guiana (A preliminary review): Institute Paper 29. Imperial forestry Institute, Oxford, United Kingdom. [ Links ]
Fearnside, P. 1997. Wood density for estimating forest biomass in Brazilian Amazonia. For. Ecol. Manage. 90: 59-87. [ Links ]
Flores, S. & N. Dezzeo. 2005. Variaciones temporales en cantidad de semillas en el suelo y en lluvia de semillas en un gradiente Bosque-sabana en la Gran Sabana, Venezuela. Interciencia 30: 39-43. [ Links ]
Fölster, H. 1992. Holocene autochthonous forest degradation in Southeastern Venezuela, 25-44. In J.G. Goldammer (ed.). Tropical forests in Transition. Birkhäuser, Basel, Basel Swiss. [ Links ]
Fölster, H., N. Dezzeo & J. Priess. 2001. Soil-vegetation relationship in base-deficient premontane moist forest-savanna mosaics of the Venezuelan Guayana. Geoderma 104: 95-113. [ Links ]
Gentry, A.H. 1988. Changes in plant community diversity and floristic composition on environmental and geographical gradients. Ann. Mo. Bot. Gard. 75: 1-34. [ Links ]
Gentry, A. 1995. Patterns of diversity and floristic composition in Neotropical montane forests, p 103-126. In S.P Churchill, H Balslev, E Forero & J.L. Luteyn (eds.). Biodiversity and conservation of Neotropical montane forests. The New York Botanical Garden, New York, USA. [ Links ]
Global Forest Watch. 2002. The State of Venezuela’s Forests: A Case Study of the Guayana Region. A Global Forest Watch report prepared by M.P. Bevilacqua, L. Cárdenas, A. Flores, L. Hernández, E. Lares, A. Mansutti, M. Miranda, J. Ochoa, M. Rodríguez & E. Selig. Global Forest Watch, Word Resource Institute, Fundación Polar, Washington, USA. [ Links ]
González, V. 2006. Los bosques de Mora gonggrijpii de un sector de la región centro oriental del estado Bolívar, Venezuela, p. 49-50. In S. Leal, L. Delgado & H.
Castellanos (eds.). Memorias del I Congreso Internacional de Biodiversidad del Escudo Guayanés, March 20-24 2006, Santa Elena de Uairén, Venezuela. [ Links ]
Gould, W.A., G. González & G. Carrero Rivera. 2006. Structure and composition of vegetation along an elevational gradient in Puerto Rico. J. Veg. Sci. 17: 563-574. [ Links ]
Grubb, J., J. Lloyd, T. Pennington & T. Whitmore. 1963. A comparison of montane and lowland rain forest in Ecuador, I. The forest structure, physiognomy and floristics. J. Ecol. 51: 567-601. [ Links ]
Hammond, D.S. 2005. Tropical rain forests of Guiana shield: ancient forests in a modern world. CABI, Wallingford, United Kingdom. [ Links ]
Hammond, D.S. & V.K Brown. 1995. Seed size of woody plants in relation to disturbance, dispersal, soil type in wet Neotropical forests. Ecology 76: 2544-2561. [ Links ]
Hart, T. 1990. Monoespecific dominance in tropical rain forests. Trends Ecol. Evol. 5: 6-11. [ Links ]
Henkel, T.W., J. Terborgh & R.J. Vilgalys. 2002. Ectomycorrhizal fungi and their leguminous hosts in the Pakaraima Mountains of Guyana. Mycol. Res. 106: 515-531. [ Links ]
Henkel, T.W. 2003. Monodominance in the ectomycorrhizal Dicymbe corymbosa (Caesalpiniaceae) from Guyana. J. Trop. Ecol. 19: 417-437. [ Links ]
Hernández, L. 1994. Clima, bosques, 80-94. In N. Dezzeo (ed.). Ecología de la Altiplanicie de la Gran Sabana I, Scientia Guaianae 4: 25-33. Caracas, Venezuela. [ Links ]
Hernández, L. (ed.). 1999. Ecología de la altiplanicie de la Gran Sabana II. Estructura, diversidad, crecimiento y adaptación en bosques de las subcuencas de los ríos Yuruaní y Alto Kukenán. Scientia Guaianae 9: 160. [ Links ]
Hernández, L. & H. Castellanos. 2006. Crecimiento diamétrico arbóreo en bosques de Sierra de Lema. Guayana Venezolana: primeras evaluaciones. Interciencia 31: 779-786. [ Links ]
Hubbell, S.P. 2001. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton University, Princenton, USA. [ Links ]
Huber, O. 1995. Vegetation, p. 63-95. In P. Berry, B. Holst, K. Yatskievych (eds.). Flora of the Venezuelan Guayana. Missouri Botanical Garden, St. Louis and Timber, Portland, Oregon, USA. [ Links ]
Huber, O. 2006 Herbaceous ecosystems on the Guayana Shield, a regional overview. J. Biogeogr. 33: 464-475. [ Links ]
Huber, O. & G. Febres. 2000. Guía ecológica de la Gran Sabana. The Nature Conservancy, Caracas, Venezuela. [ Links ]
Huber, O. & M.N. Foster (eds.). 2003 Conservation Priorities for the Guayana Shield: 2002 Consensus. Conservation International Center for Applied Biodiversity Science. Washington, DC., USA. [ Links ]
Jackson, M.L. 1976. Análisis químico de suelos. Omega, Barcelona, España. [ Links ]
Kappelle, M. & A.D. Brown (eds.). 2001. Bosques Nublados del Neotrópico. Instituto Nacional de Biodiversidad (INBio). INBio, Santo Domingo de Heredia, Costa Rica. [ Links ]
Koch-Grunberg, T. 1917. Vom Roraima zum Orinoko, vol. 1. Dietrich Reimer, Berlin, Germany. [ Links ]
Laurance, W., S. Laurance, L. Ferrreira, J. Rankin De Merona, C. Bascon & T. Lovejoy. 1997. Biomass collapse in Amazonian forest Fragments. Science 278: 1117-1118. [ Links ]
Laurance, W.F., L. Ferreira, J. Rankin.de Merona & S.G. Laurance. 1998. Rain forest fragmentation and the dynamics of Amazonian tree communities. Ecology 79: 2032-2040. [ Links ]
Laurance, W.F., P. Delamonica, S.G. Laurance, H. Vasconcelos & T. Lovejoy. 2000. Rainforest fragmentation kills big trees. Nature: 404- 836. [ Links ]
Legendre, P., D. Borcard & P. Peres-Neto. 2005. Analyzing beta diversity: partitioning the spatial variation of community composition data. Ecol. Monogr. 75: 435-450. [ Links ]
Lieberman, D., M. Lieberman, R. Peralta & G. Hartshorn. 1996. Tropical forest structure and composition on a large-scale altitudinal gradient in Costa Rica. J. Ecol. 84: 137-152. [ Links ]
Magurran, A.E. 1988. Ecological Diversity and Its Measurement. Princeton University, Princeton, New Jersey, USA. [ Links ]
McLean, E.O. 1965. Aluminum, 985-994. In C.A. Black, (ed.). Methods of Soil Analysis. Part 2. Agronomy. [ Links ]
Méndez, S. 2006. Caracterización anatómica de la Madera, con fines de análisis dendrocronológico de 8 especies provenientes de un bosque húmedo premontano alto ubicado en la parte alta de La Escalera, Sierra de Lema, estado Bolívar. UNEG. Pasantía en Ingeniería de Industrias forestales, Upata, Venezuela. [ Links ]
Mueller-Dombois, D. & F.R. Foster. 1998. Vegetation of the tropical Pacific Islands. Springer, New York, USA. [ Links ]
Mujica, N. 2007. Determinación de la densidad de madera de especies arbóreas un bosque nublado de La escalera (Sierra de Lema). Trabajo de grado, Universidad Nacional Experimental de Guayana, Ciudad Guayana, Upata, Venezuela. [ Links ]
Muller-Landau, H.C. 2004. Interspecific and Inter-site Variation in Wood Specific Gravity of Tropical Trees. Biotropica 36: 20-32. [ Links ]
Murphy, J. & J.P. Riley. 1962. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta: 31-36. [ Links ]
Nelson, B.W., V. Kapos, J. Adams, W. Olivieira, O. Braun & I. Do Amaral. 1994. Forest disturbance by large blowdowns in the Brazilian Amazon. Ecology 75: 853-858. [ Links ]
Nepstad, D.C., I.M. Tohver, D. Ray, P. Moutinho, & G. Cardinot. 2007. Mortality of large trees and lianas following experimental drought in Amazon forest. Ecology 88: 2259-2269. [ Links ]
Oliveira, M.A., A.M.M. Santos & M. Tabarelli. 2008. Profound impoverishment of the large-tree stand in a hyper-fragmented landscape of the Atlantic forest. For. Ecol. Manage. 256: 1910-1917. [ Links ]
Ortíz, J., L. Hernández & M. Worbes. 2006. Crecimiento radial de Tachigali y Terminalia en bosques de tierra baja al sudeste de Venezuela. Acta Bot. Venez. 29: 211-234. [ Links ]
Picón, G. 1995. Rare and endemic plant species of the Venezuelan Gran Sabana. Master Thesis, University of Misouri, St. Louis, USA. [ Links ]
Pitman, N., J. Terborgh, M.R. Silman & V.P. Núñez.1999. Tree species distributions in an upper Amazonian forest. Ecology 80: 2651-2661. [ Links ]
Pitman, N., J. Terborgh, M.R. Silman,V.P. Núñez, D.A. Neill, C.E. Cerón, W.A. Palacios & M. Aulestia. 2001. Dominance and distribution of tree species in an upper Amazonian terra firme forests. Ecology 82: 2101-2117. [ Links ]
Priess, J. 1996. Wurzeldynamik und Zersetzeraktivität in Ca-defizitären Böden unter tropischem Feuchtwald, Venezuela. Gött. Beitr. z. Land u. Forstwirtschaft in den Tropen u. Subtropen, H. Göttingen, Germany. [ Links ]
Priess, J., H. Fölster & C. Then. 1999. Litter and fine-root production in three types of tropical premontane rain forest in SE Venezuela. Plant Ecol. 143: 171-187. [ Links ]
Rettenmaier, R. & H. Fölster. 1999. Dinámica de la regeneración natural, p. 108-133. In L. Hernández (ed.). Ecología de la altiplanicie de la Gran Sabana: II. Estructura, diversidad, crecimiento y adaptación en bosques de las Subcuencas de los ríos Yuruaní y Alto Kukenán. Scientia Guaianae 9. Caracas, Venezuela. [ Links ]
Richards, P. 1976. The Tropical Rain Forest: An ecological study. Cambridge University, Cambridge, United Kingdom. [ Links ]
Riina, R. 1996. El elemento fitogeografico andino en la provincia Pantepui, Región Guayana, Venezuela. Ph.D. Thesis, Universidad Central de Venezuela, Caracas, Venezuela. [ Links ]
Röhl, E. 1948. Los veranos ruinosos de Venezuela. Bol. Acad. Cs. Fis. Mat. y Nat. 31: 1-23. [ Links ]
Rollet, B. 1969. Etudes quantitatives d’ une forêt dense humide sempervirente de plaine de la Guyane Vénézuelienne. Thèse doctorat Faculté des Sciences, Toulouse, Toulouse, France. [ Links ]
Rull, V. 1991. Contribución a la paleoecología de Pantepui y la Gran Sabana (Guayana Venezolana): clima, biogeografica y ecología. Scientia Guaianae 2. Caracas, Venezuela. [ Links ]
Rull, V. 1992. Successional patterns of the Gran Sabana (Southeastern Venezuela) vegetation during the last 5000 years and its responses to climatic fluctuations and fire. J. Biogeogr. 19: 329-338. [ Links ]
Rull, V. 1999. A palynological record of a secondary sucession after fire in the Gran Sabana, Venezuela. J. Quat. Sci. 814: 137-152. [ Links ]
Rull, V. 2009. New paleoecological evidence for the potential role of fire in the Gran Sabana, Venezuelan Guayana and implications for early human occupation. Veg. Hist. Archaeobot. 18: 219-224. [ Links ]
Sanoja, E. 2004. Diagnosis y observaciones sobre la biología de Catostemma lemense, nueva bombacaceae de Venezuela. Acta Bot. Venez. 27: 83-94. [ Links ]
Sanoja, E. 2009. Lista dendrológica de los bosques montanos de La Escalera, Sierra de Lema, Edo. Bolívar, Venezuela. Acta Bot. Venez. 32: 79-111. [ Links ]
Sanoja, E. 2009b. Nueva especie de Zanthoxyllum L. (Rutaceae) de Sierra de Lema, estado Bolívar, Venezuela. Acta Bot. Venez. 32: 303-309. [ Links ]
Schoengart, J. 2003. Dendrochronlogische Untersuchungen in Überschwemmungswäldern der várzea Zentralamazoniens. Heft 149. Göttinger Beiträge zur Land- und Forstwirtschaft in den Tropen und Subropen. Georg-August Universität zu Göttingen. Göttingen, Germany. [ Links ]
Schubert, C., H. Briceño & P. Fritz. 1986. Paleoenvironmental aspects of the Caroni-Paragua river basin (Southeastern Venezuela). Interciencia 11: 278-289. [ Links ]
Smith, D.N. & T.J. Killeen. 1998. A comparison of the structure and composition of montane and lowland tropical forest in the Serrania Pilón, Beni, Bolivia, p. 681-700. In F. Dallmeier & J.A. Comiskey (eds.). Forest biodiversity in North, Central and South America, and the Caribbean. Research and Monitoring. MAB Series Vol. 21. UNESCO, Paris, France. [ Links ]
Stadtmueller, T. 1987. Los bosques nublados en el Trópico Húmedo: una revisión bibliográfica. Universidad de las Naciones Unidas. Centro Agronómico Tropical de Investigación y Enseñanza, Turrialba, Costa Rica. [ Links ]
Steyermark, J. 1966b. Contribuciones a la flora de Venezuela: Parte 5. Ptaritepui. Acta Bot. Venez. 1: 9-256. [ Links ]
Steyermark, J. & S. Nilsson 1962. Botanical novelties in the region of Sierra de Lema, Estado Bolívar: I. Bol. Soc. Venez. Ci. Nat. 23: 59-95. [ Links ]
Swenson, N.G. & B. Enquist 2007. Ecological and evolutionary determinants of a key plant functional trait: wood density and its community wide variation across latitude and elevation. Am. J. Bot. 94: 451-459. [ Links ]
Tate, G.H.H. 1932. Life zones at Mount Roraima. Ecology 13: 235-257. [ Links ]
Terborgh, J. & M. Foster. 1998. Impact of a rare storm event in an Amazonian forests. Biotropica 30: 470-474. [ Links ]
ter Braak, C.J.F. 1986. Canonical Correspondence Analysis: A New Eigenvector Technique for Multivariate Direct Gradient Analysis. Ecology 67: 1167-1179. [ Links ]
ter Steege, H. 1990. A monograph of Wallaba, Mora and Greenheart. Tropenbos Technical Series 5. The Tropenbos Foundation, Wageningen, The Neteherlands. [ Links ]
ter Steege, H. 1994. Seedling growth of Mora gonggrijpii, a large seeded climax species, under different soil and light conditions. Vegetatio 112: 161-170. [ Links ]
ter Steege, H. 2000. Plant diversity in Guyana: with recommendations for a protected areas strategy. Tropenbos Series 18. Wageningen, The Netherlands. [ Links ]
ter Steege, H. 2003. Long-term changes in tropical tree diversity studies from the Guiana Shield, Africa, Borneo and Melanesia. Tropenbos Series 22. Wageningen, The Netherlands. [ Links ]
ter Steege, H. 2006. Forest, tree- and functional diversity of the Guayana Shield region in an Amazonian perspective, p. 44-46. In S. Leal, L. Delgado & H. Castellanos (eds.). Memorias del I Congreso Internacional de Biodiversidad del Escudo Guayanés. Santa Elena de Uairén, Venezuela. [ Links ]
ter Steege, H., V.G. Jetten, A.M. Polak & M.J.A. Werger. 1993. The tropical rainforest types and soils of a watershed in Guyana, South America. J. Veg. Sci. 4: 705-716. [ Links ]
ter Steege, H. & D.S. Hammond. 1996. Forest management in the Guianas: Ecological and Evolutionary constraints on Timber Production. BOS NiEuWSLETTER 15: 62-69. [ Links ]
ter Steege, H., D. Sabatier, H. Castellanos, T. van Andel, J. Duivenvoorden, A. Adalardo de Oliveira, R. Ek, R. Lilwah, P. Maas & S. Mori. 2000. An analysis of the floristic composition and diversity of Amazonian forests including those of the Guiana Shield. J. Trop. Ecol. 16: 801-828. [ Links ]
ter Steege, H. & D.S. Hammond. 2001. Character convergence, diversity and disturbance in a tropical rain forest in Guyana. Ecology 82: 3197-3212. [ Links ]
ter Steege, H., N. Pitman, O. Phillips, H. Chave, D. Sabatier, A. Duque, J. Molino, M. Prevost, R. Spichiger, H. Castellanos, P. von Hildebrand & R. Vásquez. 2006. Continental-scale patterns of canopy tree composition and function across Amazonia. Nature 443: 444-447. [ Links ]
Thomas, G.W. 1982. Exchangeable cations, 159-166. In A.L Page, R.H Miller, D.R. Keeny (eds.). Methods of Soil Analysis. Part 1. Agronomy. [ Links ]
Tiessen, H. & J.O. Moir .1993. Characterization of available P by sequential extraction, p. 73-86. In M.R. Carter (ed.). Soil Sampling and Methods of Analysis (special publication of Canadian Society of Soil Science). Lewis, Boca Raton, USA. [ Links ]
Tuomisto, H., K. Ruokolainen, R. Kalliola, A. Linna, W. Danjoy & Z. Rodriguez. 1995. Dissecting Amazonian diversity. Science 269: 63-66. [ Links ]
Vásquez, J.A. & T.J. Givnish. 1998. Altitudinal gradients in tropical forest composition, structure and diversity in the Sierra de Manantlán, Jalisco, México. J. Ecol. 86: 999-1020. [ Links ]
Veillon, J.P. 1985. El crecimiento de algunos bosques naturales de Venezuela en relación con los parámetros del medio ambiente. Rev. For. Ven. 19: 1-123. [ Links ]
Veloso, H.P., L. Góes Filho, P.F. Leite, S. Barros-Silva, H. Castro de Ferreira, R.L. Loureiro & E.F. Moura de Terezo. 1975. ‘Vegetação’, p. 307-403. In Levantamento de recursos naturais. Vol. 8. Ministério das Minas e Energia, Rio de Janeiro, Brazil. [ Links ]
Vila, M.A. 1975. Las sequías en Venezuela. Fundacomún, Caracas, Venezuela. [ Links ]
Walkey, A & I.A. Black. 1934. An examination of the Degtjareff method for determining soil organic matter and proposed modification of the cromic acid titration method. Soil Sci. 37: 29-38. [ Links ]
Weaver, P. 2000. Environmental gradients affect forest structure in Puerto Rico´s Luquillo Mountains. Interciencia 25: 254-259. [ Links ]
Weiblen, G. 2002. Canopy position and host use by hemiepihytes in Neotropical forests, p. 131-142. In G. Orians & E. Deinert (eds.). Advanced Comparative Neotropical Ecology 5 Sept-15 Nov, 2001 Costa Rica, Panama, Peru, Brazil Organization for Tropical Studies Seattle, Washington, USA. [ Links ]
Wehrden von, H., J. Hanspach, H. Bruelheide & K. Wesche. 2009. Pluralisms and diversity: trends in the use and application of ordination methods 1990-2007. J. Veg. Sci. 20: 695-705. [ Links ]
Whitmore, T.C. 1990. An introduction to tropical rain forests. Oxford University, Clarendon, Oxford, United Kingdom. [ Links ]
Whitmore, T.C. 1989. Canopy gaps and the two major groups of forest trees. Ecology 70: 536-538. [ Links ]
Williams, A., A. Dall Antonia, V. Dall Antonia, J. Almeida, F. Suárez, B. Lebmann & A. Malhado. 2005 The drought of the century in the Amazon basin: An analysis of the regional variation of rainfall on South America in 1926. Acta Amaz. 35: 231-238. [ Links ]
Worbes, M. 1999. Degradación e historia de la vegetación boscosa, p. 84-106. In L. Hernández (ed.). Ecología de la altiplanicie de la Gran Sabana. Scientia Guaianae
9. Caracas, Venezuela. [ Links ]
Meyer, R., D. David ;D. Krueger. 2004. A Minitab Guide to Statistics. Prentice-Hall, Upper Saddle River, New Jersey, USA. Downloaded: September 2008, http://www.minitab.com/de-DE/default.aspx. [ Links ]
Zanne, A.E., G. Lopez-Gonzalez, D. Coomes, J. Ilic, S. Jansen, S. Lewis, R.B. Miller, N.G. Swenson, M.C. Wiemann & J. Chave. 2009. Global wood density database. Dryad. October 2009. Identifier: http://hdl.handle.net/10255/dryad.235. [ Links ]
*Correspondencia a: Lionel Hernández, Elio Sanoja, Leandro Salazar & Hernán Castellanos; Universidad Nacional Experimental de Guayana, Apartado postal 8050, Ciudad Guayana, Estado Bolívar 8015, Venezuela; lhernand@uneg.edu.ve, eliosanoja@gmail.com, hcastell@uneg.edu.ve
Nelda Dezzeo; Centro de Ecología, IVIC, Apartado 21827, Caracas 1020-A, Venezuela; ndzzeo@ivic.gov.ve
1. Universidad Nacional Experimental de Guayana, Apartado postal 8050, Ciudad Guayana, Estado Bolívar 8015, Venezuela; lhernand@uneg.edu.ve, eliosanoja@gmail.com, hcastell@uneg.edu.ve
2. Centro de Ecología, IVIC, Apartado 21827, Caracas 1020-A, Venezuela; ndzzeo@ivic.gov.ve
Received 04-II-2011. Corrected 20-VI-2011. Accepted 22-VII-2011