Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.59 n.2 San José Jun. 2011
Bacterial infection of mudfish Clarias gariepinus (Siluriformes: Clariidae) fingerlings in tropical nursery ponds
Gabriel Ikpi & Benedict Offem
Department of Fisheries, Faculty of Agriculture, Cross River University of Technology, Obubra Campus, Cross River State, Nigeria; benbeff06@yahoo.com, gikpi@yahoo.com
Dirección de correspondencia.
Abstract
Bacterial infection among the most common cultured mudfish Clarias gariepinus in Africa, has become a cause of concern, because it constitutes the largest economic loss in fish farms. In order to provide useful biological data of the pathogens for good management practices, samples were collected monthly between January 2008 and December 2009 in three monoculture nursery ponds, located in three different positions: upriver (A, grassland), mid-river (B, mixed forest and grassland) and downriver (C, rainforest) along 200km length of Cross River floodplains, Nigeria. A total of 720 fingerlings between 15.1 and 20.7g were analyzed to determine the degree of infection. The bacterial pathogens were taken from their external surfaces, and were isolated and identified by standard methods. The caudal fins of fingerlings from pond A had the highest bacterial load (5.8x103cfu/g), while the least counts (1.2x103cfu/g) were identified on the head of fish from pond C, with Flexibacter columnaris as the major etiological agent. Pseudomonas fluorescens, Aeromonas hydrophila, Escherichia coli, Staphylococcus aureus and Micrococcus luteus were identified as co-isolates with P. fluorescens as dominant (0.7x102cfu/mL) co-isolates in pond water. Clinical signs of five white spots with red periphery appeared on the external surface of infected fish. All the fish sampled, died after 4 to 9 days. There was no significant difference in the bacterial counts between different ponds, but the difference between fish organs/parts examined was significant. Fish from these ponds are therefore potentially dangerous to consumers and highly devalued, with the economic impact to producers. Preventive methods to avoid these infections are recommended. Rev. Biol. Trop. 59 (2): 751-759. Epub 2011 June 01.
Key words: Clarias gariepinus fingerlings, Flexibacter colummnaris, bacteriogical examination, fish farm.
Resumen
Las infeccines bacterianas son comunes en el pez de cultivo Clarias gariepinus, el cual es el más cultivado en Africa y se han convertido en una causa de preocupación, ya que constituye la mayor pérdida económica en las granjas piscícolas. Se proporcionan datos biológicos de los agentes patógenos con el fin de proporcionar información útil para buenas prácticas de gestión en las granjas. Las muestras fueron recolectadas mensualmente entre Enero 2008 y Diciembre 2009 en tres viveros de estanques de monocultivo, situados en tres posiciones diferentes: río arriba (A, pastizales), mitad del río (B, bosque mixto y pastos) y aguas abajo (C, bosque) a lo largo de 200km de longitud en las llanuras de inundación del río Cross, Nigeria. Un total de 720 alevines de entre 15.1 y 20.7g fueron analizados para determinar el grado de infección. Los patógenos bacteriales fueron tomados de las superficies externas, y fueron aislados e identificados por métodos estándar. Las aletas caudales de los alevines del estanque A tuvieron la mayor carga bacteriana (5.8x103cfu/g), mientras el menor conteo de bacterias (1.2x103cfu/g) fue identificado en la cabeza de los peces del estanque C, con Flexibacter columnaris como el agente etiológico más importante. Pseudomonas fluorescens, Aeromonas hydrophila, Escherichia coli, Staphylococcus aureus y Micrococcus luteus se identificaron como co-aislamientos con P. fluorescens, como dominantes (0.7x102cfu/mL) co-aislados en el agua del estanque. Los signos clínicos fueron cinco puntos blancos con la periferia roja y aparecieron en la superficie externa de los peces infectados. Todos los peces de la muestra, murieron después de 4 a 9 días. No hubo diferencia significativa en los recuentos bacterianos entre los diferentes estanques, pero la diferencia entre los órganos y las partes de los peces examinados fue significativa. Los peces de estos estanques son potencialmente peligrosos para los consumidores y con alta devaluación, con un impacto económico para los productores. Se recomiendan métodos de prevención para evitar estas infecciones.
Palabras clave: alevines de Clarias gariepinus, Flexibacter colummnaris, análisis bacteriológico, pez de cultivo.
Clarias gariepinus (Burchell, 1822) is a species of great economic importance in Africa and South-East Asia, especially as a food fish and vital in the local sustainability of the aquaculture activity (Teugels 1986, Venden Bossiche & Bernacsek 1990). Their aquaculture attributes include ability to withstand handling stress, disease resistance, high growth rate, fecundity and palatability. There is acute reduction of these species in inland natural water bodies in Nigeria because of the over-exploitation methods of indigenous fishers that destroy the habitat and fisheries resources (Viser 1970). Nowadays, the effort made by the Nigerian government to conserve and propagate the aquaculture of this species is being hindered, since there is little information available to producers, on the species ecology and disease issues in Nigerian waters (Fagbenro et al. 1993, Okaeme & Obiekezie 1990). Studies conducted by Ugwuzor et al. (1990), Ogbondeminu et al. (1991), Ogbondeminu (1993), Ikpi & Offem (2008), revealed incidence of Flexibacter columnaris, Pseudomonas sp., Aeromonas sp., Vibrio sp., enterobacteriaceae and Gram positive bacteria as common fish pathogens responsible for different bacterial diseases in fish farms in Nigeria.
Fedoruk (1981), Plumb & Olah (1984), Hanson & Grizzle (1985), MacMillan & Tucker (1985), Noga (2000) revealed that Flexibacter collumnaris and many other bacterial diseases of fish, have a patho-physiological background related to environmental stressors. Examples of such stressors might be: husbandry factors like crowding (Ventura & Grizzle 1987), capture and hauling, water related problems like toxicants (Tucker et al. 1984, Faisal et al. 1988), temperature and oxygen extremes (Walters & Plumb 1980, Plumb & Olah 1984), transport (Blazer 1992) and rapid environmental changes (Ciembor et al. 1995).
The prevalence of infectious diseases depends on the interaction between fish pathogen and the environment (Klesius 1992, Mqolomba & Plumb 1992, Boon & Huisman 1996, Noga 2000). Besides, the pathogen spreading, mostly under unpredictable circumstances, can result in a sudden onset of a disease in an obviously healthy population.
This investigation was developed after the outbreak of an uncommon disease of fish fingerlings purchased from the fish farms under investigation, also had clinical signs on their external surface. The study therefore includes isolation and identification of the bacterial pathogens and determination of the degree of infection of the fingerlings and to advice on management.
Materials and Methods
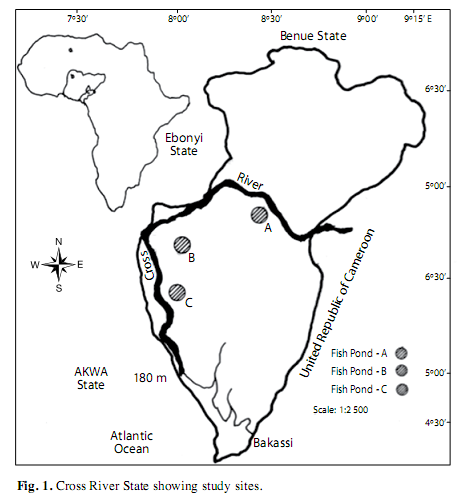
Study area: The study site is the Cross River floodplain located at the Southeastern part of Nigeria (4º.25’ - 7º.00’ N; 7º.15’ - 9º.30’ E) (Fig. 1). It is bounded in the South by the Atlantic Ocean, East by the Republic of Cameroun, the Nigerian states of Benue in the North, Ebonyi and Abia in the West and Akwa Ibom in the Southwest. Climate of the study area is characterized by a dry season from November-March and a wet season from April-October. Highest precipitation (3 050±230mm) occurs in August, and lowest (300±23mm) in March.The mean annual temperature ranged from 15.5±7.6оC in wet period to 32.6±5.4оC in dry period. As obtained in river-floodplain system Cross River comprised a wide range of habitat types, from small upland streams, smooth glides of the middle sections, and broad, meandering river stretch, of the lowland. The principle driving force for the productivity of major biota in Cross River floodplain systems is the seasonal variation of the river flows or “flood pulse,” which produces periodic inundations of the floodplain. The bulk of the productivity is derived directly or indirectly within the floodplain itself. In this study, the three ponds (A, B, C) investigated were randomly sampled, each from the floodplains upriver, mid-river and downriver. The pond sites were located 18km apart with dimensions of 30x10 x1.7m (A), 15x10x1.5m (B) and 20x10x1.5m (C).
Sample Collection: Samples were collected monthly (8am-10am) between January 2008 and December 2009 from three monoculture nursery ponds located in three reaches; A: upriver (grassland), B: mid-river (mixed forest and grassland) and C: downriver (rainforest) along 200km length of Cross River floodplains, Nigeria. A total of 720 Clarias gariepinus fingerlings of weights between 15.1 and 20.7g were sampled. Samples were held in six hapas (a net-like container used to confine fish within its natural environment for experimental purposes) placed in each of the three different fish ponds. The six fish were placed in the hapas, one in each hapa. The size of hapa was 30x15x10cm3. Specimens were transported to the fisheries laboratory and held one in each rectangular glass aquaria with volume capacity of 35x26cm3. Sampling of fingerlings in the three fish ponds was achieved using a table of random numbers, as described by Akindele (1989). All samples were then fixed in 10% formalin, and later preserved in 70% ethanol, and deposited in the Fisheries Museum (Catalogue No.: CRUTECH 1090) at the Fisheries Department of Cross River University, Obubra Campus, Nigeria.
Sampling methodology: As stated, fingerlings where held in hapas and observed until appearance of clinical signs. The specimens that showed early lesions were sampled as described in Noga (2000). The same process was repeated in fingerlings held in aquaria in the laboratory after a four hours acclimatization period. Areas examined for bacterial infection in fish to determine pathogenicity were the gills, head, body and caudal fin. They were all observed during the period of mortality.
Bacteriological examination: The examination was conducted to isolate, identify and confirm bacterial isolates. Early lesions were aseptically inoculated for culture on Cytophaga Agar (CA) medium. The inoculated media was cultured at 25oC for 18 to 48 hr. A random selection of colonies from various samples, were re-streak into fresh agar relates to ensure purity. Pure cultures of the bacterial organisms were identified using the standard procedures (Barrow & Feltham 1993). The test employed for the identification of isolates, was the Gram stain, mobility test, biochemical test, sensitivity analysis, pigments and colony morphology. The process was repeated for co-isolation of other bacterial species with samples of other external surfaces without lesions and the pond water. Co-isolations were aseptically inoculated for culture on Trypticase Song Agar (TSA) and McConkey Agar (McC). The inoculated media was cultured at different temperatures of 25 and 35oC for 18 to 48 hr.
Biochemical and sensitivity test: Biochemical tests using Cytophaga agar (CA) and Tripticase Song agar (TSA) were made following the procedures described by Roberts (1989) and Inglis et al. (1994). The culture medium was autoclaved for 15 min at 121oC and 115Hg. Cooled slants were inoculated with bacterial culture suspension by streaking slant and stabling bull. They were incubated at between 25oC and 35oC for 18 to 48 hr. Sensitivity test was carried out to confirm the isolation of Flexibacter columnaris. The antibiotic used was Penicillin.
Bacterial enumeration: Total viable count (TVC) of bacteria from the external surface of Clarias gariepinu fingerlings and pond water were determined on Cytophoga Agar (CA). Trypticase Song Agar (TSA) and McConkey Agar (McC) by the plate count technique (Roberts 1989, Inglis et al. 1994). Colony forming units (cfu) were counted with a Gallenkamp colony counter. Estimation of the bacterial populations was reported as Cfu/mL or Cfu/g of sample.
Water quality tests: The water quality parameters of the ponds and the aquaria tanks included: temperature, pH, dissolved oxygen, total suspended solids, total hardness and conductivity were determined following the method described by APHA (1980). Water used in the aquaria, was tap water acclimated for three days before use.
Data obtained from bacterial counts made from the external surface and parts of fish, pond water and aquaria were analyzed using descriptive statistics. In addition, analysis of variance was used to compare bacterial counts in the fish ponds and aquaria, as well as among the various parts of the fish. The statistical method used in the analysis was the Complete Randomize Block Design (CRBD)
Results
Pathogenicity and clinical symtoms: The results showed that Flexibacter columnaris species was pathogenic as clinical signs were observed in the external surface of fingerlings in the different fish ponds and suspected fish held in the aquarium. The clinical signs were observed in the gills, head, body and caudal fin (Table 1). The clinical signs on the external surface of Clarias gariepinus fingerlings were white spots, white spots with red periphery, lesions and death of fish specimens (Table 2 and 3). The number of white spots and white spots with red periphery were between one to five for fish specimens from the different fish ponds, and between one to three for fish held in aquarium. Lesions ranged between one to three, and one to two for fish in fish ponds and aquaria, respectively. There was a 100% mortality for fish samples in the different fish ponds and aquaria, but there was a significant (p<0.05) difference among the rate of mortality between ponds and aquaria. Nevertheless, no lesions on the head of the aquarium fingerlings were observed. The period of time, between the appearance of clinical signs on fish and mortality, varied in the different fish ponds.
Bacterial load/counts: Bacterial load ranged from 1.2x103 to 5.8x103cfu/g in the fish ponds samples, and from 1.1x103cfu/g to 4.1x103cfu/g in aquaria samples (Table 1). The caudal fin of specimens from pond A and aquaria A had the highest bacterial load of 5.8x103cfu/g and 4.1x103cfu/g respectively; while the lowest counts were obtained from the fish head (1.2x103cfu/g) of fish from pond C, and gills (1.1x103cfu/g) of fish from aquariam. No bacterial infection on the head of fish from aquaria C was observed. There was no significant difference (p>0.05) between log bacterial counts in fish from different fish ponds but the difference between log counts for different fish organs/parts was significant (p<0.05).
Co-isolation of F. columnaris with other bacteria: Five different bacterial species were isolated and identified from pond water and fish body. The bacterial species were Pseudomonas flourescens, Aeromonas hydrophila, Staphylococcus aureus, Micrococcus luteus and Escherichia coli (Table 4). The dominant co-isolated bacterial species in the pond water and the fish body of the three ponds was P. flurescens in pond A, with a bacterial load of 0.7x102cfu/mL and 0.3x102, respectively.
Biochemical properties of F. columnaris: F. columnaris is a Gram-ve bacteria and reacted positively with Cytochrome Oxidase, Catalase, Voges-Proskauer reaction, H2S production, Nitrite reduction and negatively to Indole and oxidative/fermentative reaction. Its sensitivity analysis showed that, it was sensitive to Penicillin. Examination of wet mounts from lesions showed gliding motion.
The biochemical properties for the co-isolates showed that P. fluorescens, A. hydrophila and E. coli are all Gram-ve bacteria while S. aureus and M. luteus are Gram+ve bacteria. P. fluorescens, A. hydrophila and M. luteus, reacted positively with Cytochrome oxidase, Catalase and Nitrite reduction oxidative/fermentative reaction, Vogues Proskauer and H2S production. Although P. fluorescens reacted negatively with H2S production and oxidative/fermentative reaction, E. coli and S. aureus reacted negatively with Cytochrome oxidase, Vogues-Proskauer, and H2S production. However, S. aureus showed positive reaction with Vogues-Proskauer and Nitrite reduction.
Motility test showed that M. luteus and S. aureus are non-motile while P. fluorescens, A. hydrophila and E. coli are motile.
Water quality parameters recorded during the study were temperature, pH, Dissolved Oxygen, total suspended solids, total hardness and conductivity (Table 5).
Discussion
The study showed that Flexibacter columnaris is a Gram-ve bacteria, and was pathogenic with the appearance of clinical signs on the external surface and gills, followed by the eventual death of the fish specimens in four to six days, and six to nine days, at temperatures between 21.1 and 28.7°C for the fish farms and aquaria respectively. These observations were in accordance with those reported by Wakabayashi (1993) on the death of Weather fish within seven days at 15oC and one day at 35°C. Chowdhury & Wakabayashi (1988) reported that infection of Weather Fish with F. columnaris was also found to vary with different water quality conditions.
The outbreak of F. columnaris disease in the fish farms may have been a result of purchase of fingerlings from infected fish farms, poor husbandry practices and environmental factors (Ventura & Grizzle 1987, Ciembor et al. 1995 and Noga 2000). The appearance of white spots on the gills, head, body and caudal fin, with the later having higher numbers at the first indication of infection and the white spots surrounded by a zone of red tinge on the second day of infection were similar as those reported by Inglis et al. (1994). Bacterial counts of 5.8x103cfu/g and 4.1x103cfu/g for Clarias gariepinus fingerlings in fish farms and aquaria respectively, was higher than 3.5x103cfu/g and 2.9x103cfu/g for fish cultured in a similar experiment by Ikpi & Offem (2008). Also, gill infection was common but less severe in this study than that reported by Noga (2000), who observed that although gill infection occurred, was not common in most fishes. Lesions appeared within three and four days and were not necrotic for fish in fish farms and aquaria. Similar observations has been made by Roberts (1989), Ugwuzor et al. (1990), Olufemi et al. (1991), Inglis et al. (1994) and Noga (2000) but within 24 hours for Inglis et al.(1994) and Noga (2000).
Co-isolation of F. columnaris with other bacteria revealed five different bacterial species, which were Pseudomonas florescens, Aeromonas hydrophila, Escherichia coli, Staphylococcus aureus and Micrococcus luteus, the first three are Gram-ve while the last two bacterial species are Gram+ve with P. fluorescens being dominant. This was in accordance with Wiklund & Lönnström (1994) who co-isolated Pseudomonas anguilliseptica with four bacterial species which were Vibrio anguillarum, Aeromonas salmonicida, Pseudomonas sp. and Aeromonas sp. from different species of fish in finnish farms. Chowdhury & Wakabayashi (1990), also observed that F. columonaris successfully invaded fish in the presence of other bacterial species.
Although the mudfish Clarias gariepinus is known to be infectious-disease resistant, with the increasing trend of catfish culture in this region without embarking on a training programme for local fish farmers, that seem to have difficulties in the dynamics of good aquaculture management practices.
Acknowledgment
We sincerely thank the Dean of Faculty of Agriculture and Forestry, Cross River University of Technology, Obubra Campus, for allowing us the use of part of the facility and research grants to carry out this work.
References
Akindele, S.O. 1989. Basic experimental designs in agricultural research. Montem, Akure, Nigeria. [ Links ]
APHA. American Public Health Association. 1980. Standard Methods for the Examination of Water and Wastewaters. American Public Health Association, Washington D.C., USA. [ Links ]
Barrow, G.I. & R.K.A. Feltham. 1993. Cowan and Steel’s Manual for the Identification of Medical Bacteria. Cambridge, Cambridge, England. [ Links ]
Blazer, V.S. 1992. Nutrition and disease resistance. Ann. Rev. Fish Dis. 1: 309-323. [ Links ]
Boon, J.H. & E.A. Huisman. 1996. Viral, bacterial and fungal diseases of Siluroidei, cultured for human consumption. Aquat. Living Resour. 9: 153-164. [ Links ]
Chowdhury, M.B.R. & H. Wakabayashi. 1988. Effects of competitive bacteria on the survival and infectivity of Flexibacter columnaris. Fish Pathol. 24: 9-15. [ Links ]
Chowdhury, M.B.R. & H. Wakabayashi. 1990. Effects of co-existing bacteria on Flexibacter columnaris infection in loach Misgurnus anguillicaudatus, p. 651-654. In T. Hirano & I. Hanyu (eds.). The Second Asian Fisheries Forum. Asian Fisheries Society, Manila, Philippines. [ Links ]
Ciembor, P.G., V.S. Blazer, D. Dawe & E.B. Shotts. 1995. Susceptibility of channel catfish to infection with Edwardsiella ictaluri: Effect of exposure methods. J. Aquat. Anim. Health 7: 132-140. [ Links ]
Fagbenro, O.A., C.O. Adedire, E.O. Owoseni, E.O. Ayotunde. 1993. Studies on the Biology and Aquacultural Potential of Feral Catfish, Hetrobranchus bidosalis (Geoffroy St. Hilare 1809). (Clariidae). Trop. Zool. 6: 67-79. [ Links ]
Faisal, M., E.L. Cooper, M. El-Mofty & M.A. Sayed. 1988. Immunosupressor of Clarias lazera (Pisces) by a mulluscicite. Develop. Comp. Immunol. 12: 85-97. [ Links ]
Fedoruk, A.N. 1981. A management perspective on the stress and infectious diseases in Clarias farming. National Inland Fisheries Institute, Bangkok, Thailand. [ Links ]
Hanson, L.A. & J.M. Grizzle. 1985. Nitrite-induced predisposition of channel catfish to bacterial diseases. Progr. Fish. Cult. 47: 98-121. [ Links ]
Ikpi, G.U. & B.O. Offem. 2008. Bacterial infection of cultural fishes is the fish farm of the Cross River University of Technology. Egyp. J. Microbiol. 21: 57-63. [ Links ]
Inglis, V., R.J. Roberts & N.R. Bromage. 1994. Bacteria diseases of fish. Blackwell, London, England. [ Links ]
Klesius, P. 1992. Carrier state of channel catfish infected with Edwardsiella ictaluri. J. Aquat. Anim. Health 4: 227-230. [ Links ]
MacMillan, J.R. & C.S. Tucker. 1985. Infectious diseases in channel catfish culture. Dev. Aquacult. Fish. Sci. 15: 405-496. [ Links ]
Mqolomba, T.N. & J.A. Plumb. 1992. Longevity of Edwardsiella ictaluri in the organs of experimentally infected channel catfish, Ictalurus punctatus. Aquaculture 101: 1-6. [ Links ]
Noga, E.J. 2000. Fish disease diagnosis and treatment. Iowa State University, Iowa, USA. [ Links ]
Ogbondeminu, F.S. 1993. Bacterial flora associated with the production of mudfish (Clarias anguillaris L.) in a hatchery in Bio. Res. Comm. 5: 33-38. [ Links ]
Ogbondeminu, F.S., C.T. Madu & A.N. Okaeme. 1991. Bacteriological aspects of cultured fingerlings of Clarias anguillaris L. in a hatchery complex in Nigeria. J. Aqua. Trop. 6: 45-54. [ Links ]
Okaeme, A.N. & A.O. Obiekezie. 1990. Some emergent Diseases and Management Problems of Oreochromis niloticus, Sarotherodon galileus and Clarias species in Nigeria J. Aquat. Sci. 2: 153-161. [ Links ]
Olufemi, B.E., D.A. Akinlabi & S.A. Agbede. 1991. Aerobic bacterial Pathogens isolated from the African Catfish Clarias gariepinus (Burch). Trop. Vet. 9: 177-180. [ Links ]
Plumb, J.A. & J. Olah. 1984. Pathogens and environment in European Polyculture. Relationship of Water quality and infectious diseases in cultured channel catfish. Symp. Biol. Hung. 23: 182-199. [ Links ]
Roberts, R.J. 1989. The Pathophysiological and Systemic Pathology of teleosts, p. 56-134. In R.J. Roberts (ed.). Fish Pathology. Bailliere Tindall, London, England. [ Links ]
Tucker, C., S. MacMillan & T.E. Schwedler. 1984. Influence of Edwardsiella ictaluri septicemia on nitrite-induced methaemoglobinaemia in channel catfish (Ictalurus punctatus). B. Environ. Contam. Tox. 32: 669-673. [ Links ]
Teugels, G.G. 1986. A systematic revision of the African species of the genus Clarias (Pisces: Alariidae). Koninklijk Museum Voor Midden-Africa Tervuren, Belgie. Zool. Weten. Ann. 247: 35-41. [ Links ]
Ugwuzor, N.G., D.L. Anadu & C. Ejike. 1990. Pseudomonad infection of catfish of the genus Clarias gariepinus (Teugels 1984). J. Aquat. Sci. 5: 11-13. [ Links ]
Ven den Bossche, J.P. & G.M. Bernacsek. 1990. Source book for the inland fisheries resources of Africa 2. CIFA Technical paper 18.2. FAO Fisheries Department, Rome, Italy. [ Links ]
Ventura, M.T. & J.M. Grizzle. 1987. Evaluation of Portals of entry of Aeromonas hydrophila in channel catfish. Aquaculture 65: 205-214. [ Links ]
Viser, S.A. 1970. Kainji, a Nigerian man-made lake. Kainji Lake studies. Nigerian Institute of Social and Economic Research, Ibadan. [ Links ]
Wakabayashi, H. 1993. Columnaris disease, p. 23-39. In V. Inglis, R.J. Roberts & N. Bromage (eds.). Bacterial Diseases of Fish. Wiley-Blackwell, New York, USA. [ Links ]
Walters, G.R. & J.A. Plumb. 1980. Environmental stress and bacterial infection in Channel Catfish, Ictalurus punctatus Rafinesque. J. Fish Biol. 17: 177-185. [ Links ]
Wiklund, T. & L. Lönnström. 1994. Occurrence of Pseudomonas anguilliseptica in Finnish fish farms. Aquaculture 126: 211-217.
[ Links ]
Correspondencia a: Gabriel Ikpi & Benedict Offem: Department of Fisheries, Faculty of Agriculture, Cross River University of Technology, Obubra Campus, Cross River State, Nigeria; benbeff06@yahoo.com, gikpi@yahoo.com
Received 29-I-2010. Corrected 01-XI-2010. Accepted 14-XII-2010.