Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.58 suppl.3 San José Oct. 2010
The distribution of seagrasses in Dominica, Lesser Antilles
S.C.C. Steiner, K.J. Macfarlane, L.M. Price & D.A. Willette
Institute for Tropical Marine Ecology Inc. (ITME), P.O. Box 944, Roseau, Commonwealth of Dominica; admin@itme.orgAbstract
Key words: Seagrasses, Syringodium, Halodule, Halophila, Thalassia, Dominica.
Resumen
Pastos marinos son los ambientes más grandes constituidos por organismos en Dominica. Sin embargo, sólo se han examinado desde 2007. Entre el 10 de septiembre y 9 de diciembre 2008, se examinaron la composicion de especies y la densidad de magnoliofitas en profundidades de 0 a 24m. Los Cymodoceaceae: Syringodium filiforme y Halodule wrightii, tal como los Hydrocharitaceae: Halophila decipiens, H. stipulacea y Thalassia testudinum,mostraron una distribución regional y horizontal muy distinta. Syringodium filiforme fue la especie dominante en las costas del oeste y del norte de la isla. Se encontró en profundidades de 2 a 18m y con un promedio de cobertura béntica de 0.9-10% en la costa del oeste. En las costas del norte creció entre 0.2 y 1m de profundidad con un pormedio de cobertura béntica de 48.9%. Halodule wrightii creció en las costad del norte y oeste, en profundidades de 1 a 14m en áreas de perturbaciones recientes o crónicas. Su morfología delicada y su baja cobertura béntica (<0.1%) no constituyeron pastos. Halophila decipiens creció en los márgenes profundos, llanos y laterales de pastos dominados por S. filiforme, pero también en forma mono-específica entre 3 y 24m. Halophila stipulacea, una especie invasora, se encontró comúnmente a lo largo de 45km de la costa del oeste en profundidades entre 5 y 24m. Ambas especies de Halophila formaron pastos extensos en profudidades mayores al límite de este estudio y puden ser importantes en la recolonización de áreas llanas después de tormentas destructivas. H. decipiens y H. stipulacea ahora son las especies más comunes después de S. filiforme, a pesar de su ausencia en la costa del norte. T. testudinum, sólo creció en la costa del norte, encima de áreas arrecifales con un pormedio de cobertura béntica entre 2 y 76%, en aguas con 1m o menos de profundidad. Crecieron mono-específicamente en áreas de turbulencia máxima y mínima, pero entre S. filiforme en áreas de turbulencia moderada. Oleaje fuerte en la costa del oeste (octubre 16), asociado con el Huracán Omar, causó la erosión y el enterramiento variado de las márgenes llanas de pastos marinos, particularmente entre 2 y 10m de profundidad. Este evento demostró la dinámica en las márgenes llanas de pastos marinos de Dominica y el nivel de resistencia a perturbaciones de pastos individuales.Palabras clave: algas marinas, Syringodium, Halodule, Halophila, Thalassia, Dominica.
All of Dominica´s seagrass beds are close to human settlements and are historically and presently used as fishing areas, locally also referred to as "conch beds". The fisheries are artisanal and the fishing techniques applied include seine net fishing, the use of baited fish pots, spear fishing and occasionally the collection of the gastropods Strombus gigas and S. costatus and the echinoid Tripneustes ventricosus. Visible signs of stresses affecting seagrasses in Dominica are the burial and erosion of seagrasses, the contamination by solid wastes, and pulses in the colonization and growth of epiphytic organisms and macroalgae. Seagrasses are not yet specifically protected by the laws of Dominica or other conservation mechanisms as no major seagrass beds occur within the boundaries of the two existing marine protected areas, the Cabrits National Park and the Scott´s Head – Soufrière Marine Reserve. In order to expand our recent understanding of Dominica´s largest marine organism-built habitat type, and to provide the primary data based on field surveys, necessary for the sound development of environmental policies, this study examines the species composition and topography within and between seagrass beds at depths ranging from 0 to 24m.
Materials and methods
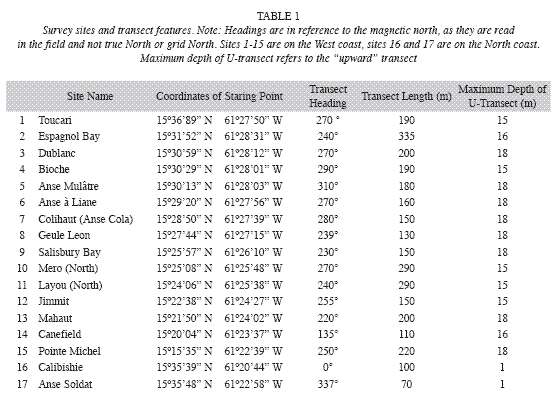
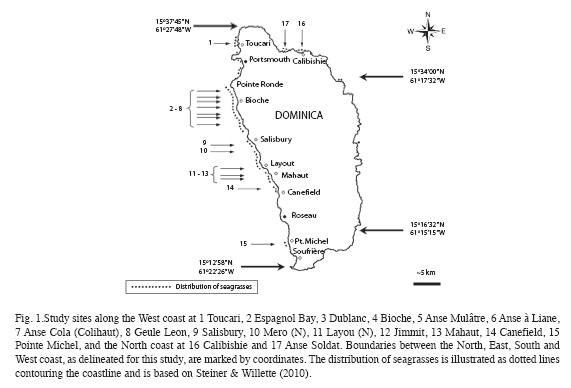
Seagrass beds at fifteen sites along the West coast and two sites along the North coast (Fig. 1) were surveyed between September 10 and December 7, 2008 (Table 1). The sites were strategically selected to encompass all major areas of seagrass beds as ascertained by Steiner and Willette in 2007. At all west coast sites SCUBA dives were carried out following a compass heading perpendicular to the shoreline. Snorkeling gear was used to examine north coast sites. The coordinates of the starting point, and the heading, length and maximum depth of each transect were recorded for future reference (Table 1). The survey protocol entailed a "downward" and an "upward" transect. During the downward transect, from shore to a depth of 24m or to where no more seagrasses were seen (whichever came first), qualitative descriptions of the seagrass bed margins (intact or eroded, species composition, height and benthic cover of leaf shoots) and the central parts (species composition, height and benthic cover of shoots, signs of endofauna, epiphytes, sessile and vagile fauna) were recorded. Seagrasses observed beyond the deep end of each transect were also noted and their benthic cover was estimated. At Espagnol Bay the deep margin of the S. filiforme bed was further than 335m from shore and was not reached during the survey. Each "upward" transect included quantitative assessments of "plots" consisting of three 1m2 quadrats, aligned perpendicular to shore and 1m apart from each other, first at a distance of 5m from the deep margin of the seagrass bed or at the deepest point of the "downward" transect if a seagrass bed margin was not reached, and repeated at 20m intervals towards shore. Intervals were measured with a 20m transect line extended in the opposite direction of the compass heading used during the "downward" transect. At Toucari and Espagnol Bay a 40m interval was used to allow for the completion of the survey during a single dive. Within each quadrat, seagrass species composition, benthic cover and height of leaf shoots were assessed and the presence of macroalgae, epiphytes, sedentary and vagile epifauna was noted. The benthic cover of leaf shoots was visually estimated using 1m2 quadrats subdivided into four 0.25m2 squares. Each estimate was derived from the consensus of at least two surveyors and was based on eight hours of consistency training in the field and with the aid of projected images, to ensure that surveyors estimated the percent cover congruently. Shoots closest to five pre-marked spots along the quadrat were selected for in situ height measurements to the closest centimeter. Quantitative data of the three quadrats constituting each of the plots were pooled and the means used for the comparison within and between seagrass beds and depth. Species identification in the field was based on Littler & Littler (2000). Voucher specimens were stored at the Institute for Tropical Marine Ecology.
Outer wind bands of Hurricane Omar caused strong swells along Dominica´s West coast during the night of October 15 and through most of the day on October 16, 2008. Field surveys were suspended due to poor visibility caused by the storm and resumed on October 23, 2008. Storm impacts in the form of erosion and burial of seagrasses in varying degrees, were evident in many seagrass beds. The width of seagrass beds and the species presence were subsequently reassessed.
Results
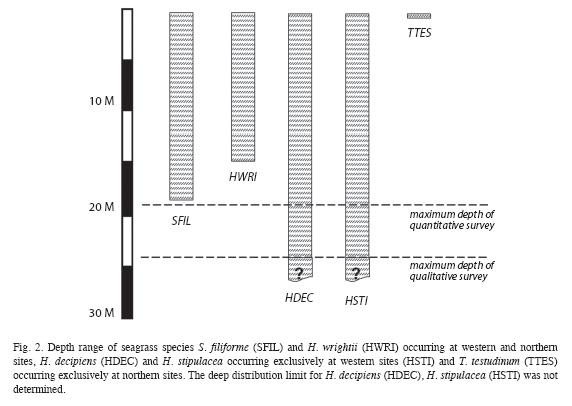
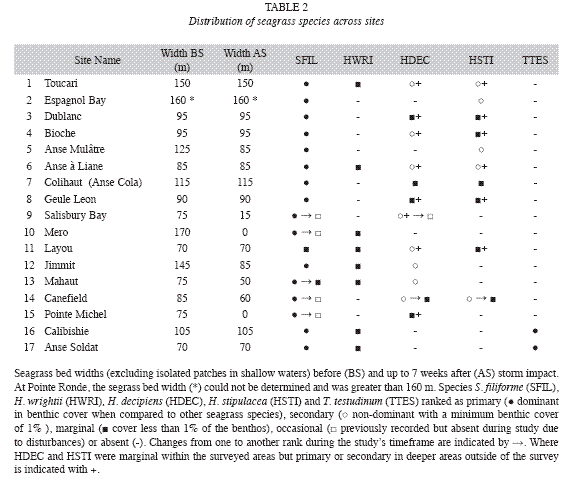
Halodule wrightii grew in depths between 1 and 14m (Fig. 2), in areas of recent (generally in shallow waters) and chronic disturbances (e.g. Layou river mouth), at western and northern sites, but did not occur in any of the 141 quantitatively examined quadrats. Its delicate morphology and sparse benthic cover (<0.1%) did not constitute seagrass beds that shape their substrate. H. decipiens grew along the deep, shallow and lateral margins of the west coast S. filiforme beds and monospecifically in depths between 3 and 24m, however it did not occur in any of the quantitatively assessed quadrats. It was most commonly found along the deep margins of S. filiforme beds forming mono-specific beds with a benthic cover of shoots of up to 30% or in variegated or mixed assemblages with H. stipulacea. The latter was also widespread (Table 2) along 45km of the West coast between Canefield and Toucari and occurred in depths from 5 to 24m, predominantly along the deep margins of S. filiforme beds and with benthic covers of up to 80%. Only at Canefield did H. stipulacea occur within the quantitatively surveyed quadrats, where its mean benthic cover of shoots was 21.4% (n=12) with a mean blade height of 6cm (n=60). Both Halophila species formed extensive beds at depths beyond the survey limit of this study at 24m (Fig. 2) where H. decipiens still covered a greater portion of the seafloor than H. stipulacea.
Thalasssia testudinum was confined to North coast where it occurred on sheltered reef flats at depths of 1m or less (Fig. 2), with a mean benthic cover ranging from 2 to 76% (n=18). It grew monospecifically in the most turbulent locations (coral rubble sediments behind the reef crest) and the calmest locations (along the shore), yet intermixed with S. filiforme in areas of moderate turbulence (central portions of the seagrass bed). At Calibishie the mean benthic cover and blade height within the seagrass beds increased steadily from the offshore margin (most turbulent) to the near-shore margin (calmest) where they peaked at 76.6% (n=3) and 22.8cm (n=15) respectively. The same pattern was observed in the mean blade heights across the narrower seagrass bed at Anse Soldat, which increased steadily from the off-shore margin (11.1cm, n=15) towards shore (22.8cm, n=15). However, the mean benthic cover had a sinusoid distribution with a mean benthic cover of 7% (n=3) at the off shore and near shore margins and peaking at 19% (n=3) in the central portions of the seagrass bed.
Discussion
H. wrightii displayed the sparsest yet most "opportunistic" distribution in that it occurred in areas of disturbance lining or within mixed and mono-specific seagrass beds, and patches of S. filiforme and T. testudinum. Furthermore, it was the first species to reappear at sites disturbed by Hurricane Omar. This is in accordance with their status as pioneer species and described by Mariani & Alcoverro (1999).
Both H. decipiens and H. stipulacea were only found along the West coast. H. decipiens is known to grow at depths of up to 40 m (Williams & Dennison 1990). H. stipulacea generally grows at depths of up to 50m (Beer & Waisel 1981), but as deep as 85 m near the Great Barrier Reef and Cargolos (den Hartog 1970). The deep distribution limit for these species in Dominica has not yet been determined, as this survey did not go beyond 24m depth. However, based on their wide distribution, in particular along the deep distribution limits of the west coast S. filiforme beds, first examined during this study, Halophila spp. must be considered by hurricanes and also have the potential of locally resettling shallower waters after storm disturbances. Although all west coast seagrasses beds were affected by Hurricane Omar, the level of destruction varied greatly between sites. This is an indication as to which seagrass beds are more resistant to such disturbances (Espagnol Bay, Dublanc, Bioche, Colihaut), which in turn is a key insight for establishing conservation measures tailored specifically to the local conditions.
This study has discerned the differences in the seagrass species distribution of the leeward and windward coasts of Dominica, where all sublittoral ecosystems cover comparatively small areas along the island´s narrow shelf and close to coastal settlements. Dominica´s seagrass beds also undergo dynamic fluctuations in their benthic cover, resulting from storms, as observed here. In such a scenario, marine conservation measures aimed at multiple rather than single habitats (e.g. coral reefs) seem more appropriate given Dominica´s contiguous layout of seagrass beds and coral reefs described in Steiner & Willette (2010). To date, Dominica´s marine habitat conservation measures and two marine protected areas (Scott´s Head-Soufrière Marine Reserve, Cabrits National Park) focus on coral reefs and areas of coral assemblages.Although seagrasses are Dominica´s largest organism-built habitat type, their investigation has only recently begun and non-anecdotal information is now available for the incorporation into broader marine conservation efforts.
Acknowledgments
Logistic and financial support was provided by the Institute for Tropical Marine Ecology Inc. (ITME RPfa09). The research license for this project (RP-09/248-1) was granted and issued by the Fisheries Development Division, Government of Dominica.
References
Agawin, N.S.R. & C.M. Duarte. 2002. Evidence of direct particle trapping by a tropical seagrass meadow. Estuaries 25: 1205-1209. [ Links ]
Baelde, P. 1990. Differences in the structure of fish assemblages in Thalassia testudinum beds in Guadeloupe, French West Indies, and their ecological signifficance. Mar. Biol. 105: 163-173. [ Links ]
Beer, S. & Y. Waisel. 1981. Effects of light and pressure on photosynthesis in two seagrasses. Aquat. Bot. 13: 331-337. [ Links ]
Constanza, R., R. d´Arge, R. De Groot, S. Farber, M. Grasso, B. Hanon, K. Limburg, S. Naeem, R.V. O´Neill, J. Paruelo, R.G. Raskin, P. Sutton & M. van den Belt. 1998. The value of the world´s ecosystem services and natural capital. Ecol. Econ. 25: 3-15. [ Links ]
Danovaro, R. & C. Gambi. 2002. Biodiversity and trophic structure of nematode assemblages in seagrass systems: evidence for a coupling with changes in food availability. Mar. Biol. 141: 667-677. [ Links ]
Den Hartog, C. 1970. The Sea-Grasses of the World. North-Holland, Amsterdam, Netherlands. Duarte C.M. 1991. Allometric scaling of seagrass form and productivity. Mar. Ecol. Prog. Ser. 77: 289-300. [ Links ]
Duarte, C.M. 2002. The future of seagrass meadows. Environ. Conser. 29: 192-206. [ Links ]
Duarte, C.M. & C.L. Chiscano. 1999. Seagrass biomass and production: a reassessment. Aquat. Bot. 65:159-174. [ Links ]
Fonseca, M.S. & J.A. Cahalan. 1992. A preliminary evaluation of wave attenuation by four species of seagrass. Est. Coast. Shelf Sci. 35: 565-576. [ Links ]
Gacia, E., H. Kennedy, C.M. Duarte, J. Terrados, N. Marba, S. Papadimitriou & M. Fortes. 2005. Light-dependence of the metabolic balance of a highly productive Philippine seagrass community. J. Exp. Mar. Biol. Ecol. 316: 55-67. [ Links ]
Grizzle, R.E., F.T. Short, C.R. Newell, H. Hoven & L. Kindblom. 1996. Hydrodynamically induced synchronous waving of seagrasses: "monamis" and its possible effects on larval mussel settlement. J. Exp. Mar. Biol. Ecol. 206: 165-177. [ Links ]
Government of Dominica. 2005. Dominica´s Biodiversity Strategy Action Plan. Government of Dominica, Roseau, Commonwealth of Dominica. [ Links ]
Lee, K. & K.H. Dunton. 1999. Inorganic nitrogen acquisition in the seagrass Thalassia testudinum: Development of a whole-plant nitrogen budget. Limnol. Oceanogr. 44: 1204-1215. [ Links ]
Lee, K.S., S.R. Park & Y.K. Kim. 2007. Effects of irradiance, temperature and nutrients on growth dynamics of seagrasses: A review. J. Exp. Mar. Biol. Ecol. 355: 144-175. [ Links ]
Littler, D.S. & M.M. Littler. 2000. Caribbean Reef Plants. Offshore Graphics, Washington, U.S.A. [ Links ]
Mariani, S. & T. Alcoverro. 1999. A multiple-choice feeding-preference experiment utilizing seagrasses with a natural population of herbivorous fishes. Mar. Ecol. Prog. Ser. 189: 295-299. [ Links ]
Onuf, C.P., R.C. Phillips, C.A. Moncreiff, A. Raz-Guzman & J.A. Herrera-Silveira. 2003. The seagrasses of the Gulf of Mexico, p. 224-233. In E.P. Green & F.T. Short (eds.). World Atlas of Seagrasses. University. Of California, Berkeley, California, U.S.A. [ Links ]
Peterson, B.J. & K.L. Heck. 2001. Positive interactions between suspension-feeding bivalves and seagrass – a facultative mutualism. Mar. Ecol. Prog. Ser. 213: 143-155. [ Links ]
Randall, J.E. 1965. Grazing effect on sea grasses by herbivorous reef fishes in the West Indies. Ecology 46: 255-260. [ Links ]
Steiner, S.C.C. & D.A. Willette. 2010. The distribution and size of benthic marine habitats in Dominica, Lesser Antilles. Rev. Biol. Trop. 58: 589-602. [ Links ]
UNEP-WCMC. 2007. (Downloaded: 21 December, 2009,www.stort.unep-wcmc.org/imaps/marine/ seagrass/viewer.htm). [ Links ]
Williams, S.L. & W.C. Dennison. 1990. Light availability and growth of a green macroalga (Caulerpa cupressoiudes) and a seagrass (Halophila decipiens). Mar. Biol. 106: 437-443. [ Links ]
Correspondencia: S.C.C. Steiner, K.J. Macfarlane, L.M. Price & D.A. Willette. Institute for Tropical Marine Ecology Inc. (ITME), P.O. Box 944, Roseau, Commonwealth of Dominica; admin@itme.org
Received 15-VII -2009. Corrected 07-II -2010. Accepted 12-VII -2010.