Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.51 n.1 San José Mar. 2003
Abstract
The physico-chemical characteristics and phytoplankton of Awba reservoir in the University of Ibadan, Nigeria, were monitored to determine the impact of eutrophication on phytoplankton composition. The principal component analysis identified three major components influencing the physicochemistry of the water, namely trace metals, dissolved oxygen and ionic composition. Comparative analysis with a previous study showed a phenomenal increase in zinc, copper and iron levels over a 10-year period. Furthermore, the ferruginous nature of the soil contributed to the high levels of iron which exceeded the World Health Organization Standards for drinking water quality. The most abundant phytoplankton species was Microcystis aeruginosa which has been implicated in toxic blooms in freshwaters. The conditions favouring cyanophyte blooms and their implications are discussed.
Key words: Awba reservoir, phytoplankton, cyanophyte blooms, trace metals, Nigeria.
Limnological studies on water bodies in West Africa have focussed on large rivers such as river Sokoto (Holden and Green 1960), river Senegal (Van de Velde 1978), river Jong (Wright 1982), the Volta river system (Biney 1990) and lakes such as the West Cameroonian crater lakes (Green 1972), lake Sonfon (Green 1979), lake Kainji (Bidwell and Clarke 1977), lake Chad (Carmouze et al. 1983) and the Niger lakes (Segers et al. 1992). However, many smaller lakes and reservoirs abound whose study would contribute significantly to the understanding of tropical freshwater ecosystems and the impact of anthropogenic activities on them (Araoye 2002). Since smaller lakes and reservoirs are more susceptible to human impact, these systems require close monitoring and evaluation.
The spatio-temporal ranges of phytoplankton species has led to the habitat-template approach which proposes that algal attributes are matched to opportunities provided by the environment and that there is an encouraging fit of phytoplankton species to the range of habitats described by the trophic spectrum. Certain assemblages are thus referred to as oligotrophic species (desmids, chrysophytes, diatoms) or eutrophic indicators (cyanobacteria, euglenoids) (Reynolds 1998). The excessive abundance or blooming of eutrophic species has detrimental effects on the domestic, industrial and recreational uses of water and is in many cases a direct motivation for restorative measures (Bryant 1994).
However, it is not valid to ascribe phyla or classes exclusively to one part of the trophic range as diatoms, chlorophytes and cyanophytes occur right across the width of the spectrum from ultraoligotrophy to hypereutrophy. Rather what is needed is a basis for relating the differing arrays of adaptive attributes and trade-offs of individual species of phytoplankton and how these might interact with the totality of processes which together determine their pelagic environments.
For over 25 years, research has been conducted on aspects of the ecology of Awba reservoir e.g. trace metal levels (Mombeshora et al. 1981, 1982), reproductive biology of the fish (Omotosho 1985), food and feeding relationships (Ugwumba and Adebisi 1992). Presently, there is a deterioration in water quality of the reservoir due to effluent discharge and eutrophication (Oduwole 1990). This paper reports on physico-chemical characteristics, trace metal levels and phytoplankton composition in the reservoir. This is important because the reservoir serves as an alternative source of water supply to the University community in the dry season months (Nov – May) when there is water shortage.
Materials and methods
Study area: Awba reservoir in the University of Ibadan (7º 26 N, 3º 53 E) is located in the south western region of Nigeria about 160 km from the Atlantic Ocean coast at an altitude of 185 m above sea level. The annual rainy season occurs from April to October with a characteristic «August break» during which rainfall abates. Meteorological conditions within the University during the study period were as follows: Mean daily minimum temperature (22.1 – 24.4ºC), Mean daily maximum temperature (24.9 – 36.8ºC), Total precipitation (125.2 – 278.5 mm), Mean relative humidity at 10.00 am (76 – 88 mm). Awba reservoir is a small man-made lake with a surface area of 6 hectares and mean depth of 5.5 m. The water of the lake is still with occasional multi-directional water movements due to wind effects. Wind action in the reservoir is minimal in the dry season and the high temperatures at this period result in thermal stratification of the water.
It is surrounded by a modified tropical rain forest vegetation with aquatic macrophytes such as Pistia stratiote s, Canna indica, Nymphae lotus, Banhima sp. on its edges.
The fish fauna of the reservoir includes Heterotis niloticus, Barbus callipterus, Clarias gariepinus, Tilapia zillii, Hemichromis fasciatus, Oreochromis niloticus, Sarotherodon galilaeus, Channa obscura and Alestes longipinnis (Ugwumba and Adebisi 1992).
Four sampling stations were chosen - station A is a drain that receives waste water from the graduate hostel and sometimes sewage from a damaged septic tank nearby. Station B is at the outflow of water containing animal wastes from the Zoological garden while C, is the source of chemical effluents and is located behind the laboratories. At this site, the water is usually reddish-brown and the sandy substratum has the same colouration. Station D is located within the main reservoir and during the dry season the water surface is partially covered by the water hyacinth – Eichhornia crassipes.
Sampling and analyses: Water samples were collected fortnightly in acid-washed 2 l -1 polyethylene containers between April and October 1989. Temperature was determined insitu with a 0-50 0 C thermometer while pH and conductivity were determined using a portable Canlab pH meter and WPA conductivity meter. Dissolved oxygen was determined by Winklers titrimetric method while ammonia-nitrogen and nitrate-nitrogen were determined spectrophotometrically. Trace metals were analysed according to methods in APHA (Anonymous 1985) using a Phillips Pye-unicam Sp 9 atomic absorption spectrophotometer. Phytoplankton were collected with a 64 µm bolting silk plankton net and preserved in 4% formalin.
Data were analysed by Principal Component Analysis (PCA) using the software Statview 513+. PCA is a multivariate statistical technique which generates a sequence of variates known as components in a correlation matrix. These components are an exact mathematical transformation of the original set of variables to a new set which are orthogonal (uncorrelated) and summarize the linear relationships or variations exhibited in the data (Omori and Ikeda 1984).
Results
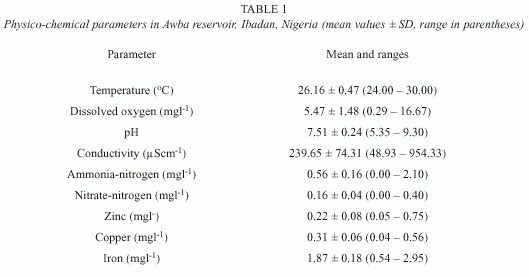
Table 1 shows the mean and ranges of physico-chemical parameters. Dissolved oxygen, pH, conductivity and ammonia-nitrogen showed wide variations during the study. The oxygen content of the reservoir is inadequate (mean 5.47 ± 1.48 mgl -1 ) and deoxygenation of the water occurred periodically. The pH is neutral (mean 7.51 ± 0.24) while conductivity recorded intermediate values (mean 239.65 ± 74.31 µScm-1). Ammonia-nitrogen values were higher than nitrate-nitrogen values (Table 1). Iron is the most abundant trace metal in the water (x = 1.87 ± 0.18 mgl -1).
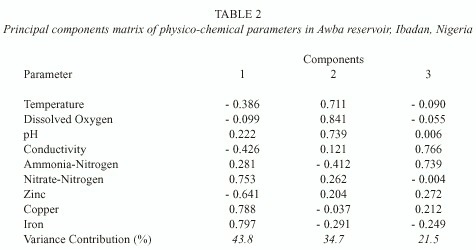
Table 2 shows the varimax-rotated components matrix of physico-chemical parameters.
Three components were selected which accounted for 100% of variance in the data. The first component accounted for 43.8% of variation and was associated with iron, copper and nitrate-nitrogen. In addition, zinc recorded a high negative loading on this component. It can be referred to as a trace metal factor. The second component was associated with dissolved oxygen, pH and temperature. It accounted for 34.7% of variation and can be referred to as a dissolved oxygen factor. The third component accounted for 21.5% of variation and was associated with conductivity and ammonia-nitrogen.
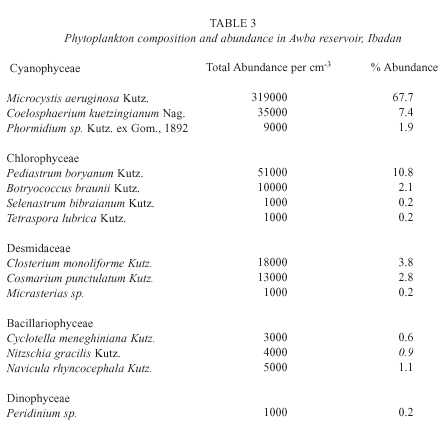
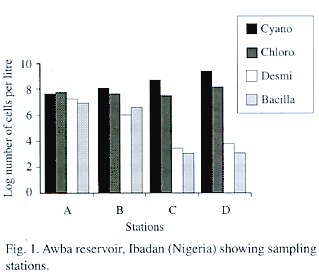
Table 3 gives the composition and abundance of phytoplankton in the study area during the investigated period. Generally, the blue-greens (Cyanophyceae) dominated the community and the most abundant species was Microcystis aeruginosa which reached a bloom proportion of 3.19 x 10 5 cells per cm -3 . There was a gradual increase in the abundance of cyanophytes fromstation A to D (Fig. 1). Contrastingly, diatoms (Bacillariophyceae) and desmids decreased from station A to D. The green algae (Chlorophyceae) were second in order of abundance and Pediastrum boryanum was the dominant species among them. Their abundance was relatively stable across stations.
The wide variations recorded in dissolved oxygen content, pH, conductivity and ammonia-nitrogen may be due to the fact that sampling took place during the late dry / rainy season periods. In tropical systems, marked variations in temperature and rainfall between seasons influence the physico-chemical characteristics of water bodies (Adebisi 1981, Chapman and Kramer 1991).
The conductivity of reservoirs and manmade lakes depend largely on that of inflowing rivers, turnover rates and the soil of the catchment area (Payne 1986). In view of these, ionic concentration in Nigerian reservoirs and lakes are highly variable with ranges such as 15 – 24 µScm-1 (Opi lake, Enugu state; Hare and Carter 1984) and 2,400 – 8,200 µScm -1 (Jakara reservoir, Kano state; Adeniji and Mbagwu 1990). From the above, the conductivity of Awba reservoir can be regarded as being intermediate. The lower nitrate-nitrogen values recorded relative to ammonia-nitrogen values has significant implications for nutrient dynamics and phytoplankton composition. The algal species that will proliferate in the reservoir must not only be able to withstand conditions of nitrogen limitation but also be able to utilize ammonia as a nitrogen source or be a nitrogen-fixing species (Raymont 1980, Paerl and Tucker 1995).
From table 4, it is apparent that there is an alarming increase in metal levels in the water.
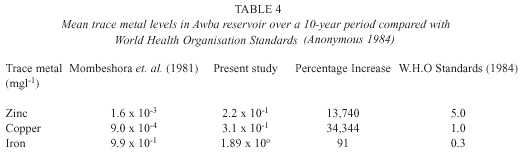
In addition, the PCA identified trace metals as the most important factor responsible for variations observed in the data. Although the levels of zinc and copper are still below the recommended limits, there is need for urgent action to prevent further increase. This is because trace metals act as micronutrients at low concentrations but at higher levels they become toxic depending on the prevalent chemical form (i.e. speciation) of the trace element in water. Also bioaccumulation and food chain magnification of trace metals may occur. Trollope and Evans (1976) reported the bioac-cumulation of metals by freshwater microflora blooms in the Swansea valley, Britain with concentration factors ranging from 10 2 – 10 5 .
However, the ferruginous nature of Ibadan soils (Aweto 1994) may account for the levels of iron which exceeded recommended limits for drinking water quality.
The stratified nature of the reservoir at the beginning of this study (late dry season) contributed to the formation of blooms. Ganf (1974) writing on water blooms caused by Microcystis aeruginosa in equatorial lake George (Uganda), drew attention to the windless (anticyclonic) weather by night which aided the formation of surface water blooms. This species was noted in lowland reservoirs (of temperate zone) at water temperatures between 21 and 25ºC and pH 8, however in submontane reservoirs it was found at lower temperatures 18ºC and pH 8.35 (Bucka and Wilk-Wozniak 1999). Hence one can say that it forms water blooms in a wide range of temperatures in all seasons of the year except winter.
According to Reuter and Petersen (1987), cyanobacteria have higher requirements for trace elements compared with eukaryotic phytoplankton hence increased levels favour their growth and physiology.
Although phytoplankton species composition and diversity changes with environmental conditions such as nutrient levels, temperature, light, predator pressure etc, the relative importance of these factors varies considerably among different taxa. Under conditions of nutrient enrichment or eutrophication, the blue-greens are known to proliferate and form noxious blooms in freshwater environments (Reynolds 1984). According to Reynolds and Rogers (1976), who studied the distribution and buoyancy of M. aeruginosa in Rostherne mere, English lake district, colonies overwinter in a vegetative form on bottom sediments and return to the epilimnion later in spring or summer. The annual growth cycle of the species is closely connected to thermal stratification in the mere. Reynolds et al. (1981, 1984b) noted that the main growth phase is the planktonic form which is photoautotrophic while the benthic form is photoheterotrophic and serves as the «inoculum» for the plankton. The development of phytoplankton blooms in eutrophic lakes is attributed to their ability to accommodate reduced nitrogen to phosphorus ratios, low edibility due to their large colony sizes coupled with large herbivore regulation of other taxa (Barica 1994, Paerl and Tucker 1995). The species recorded in Awba reservoir as that causing water bloom Microcystis aeruginosa is a gas-vacuolate, non-nitrogen-fixing cyanophyte. It can assimilate ammonia and this affords it a competitive advantage over other species under conditions of nitrogen limitation. According to Kilham and Kilham (1980) and Sommer (1981) chlorococcus green algae exhibit r developmental strategy requiring abundant nutrients, less light , high reproductive rate and short life cycles hence they cannot compete efficiently with cyanophytes which exhibit k developmental strategy and are able to survive and bloom even under conditions of high light intensities and low nutrients. Cyanophytes also exploit nutrients at the bottom of the euphotic zone by buoyancy regulation of its gas vacuoles (Reynolds 1984b). The combination of these unique physiological attributes under environmentally- favourable conditions is responsible for the proliferation and bloom of the species. The formation of blooms also depends on retention time, type and age of water body as well as calm weather conditions with low turbulence of water (Bucka 1989).
Cyanobacteria can produce both hepatotoxic peptides which cause liver damage and are tumor-favouring e.g. microcystin (produced by M. aeruginosa) as well as neurotoxic alkaloids that affect the nervous system e.g. aphanotoxin (produced by Aphanizomenon flosaqua e) and anatoxina produced by Anabaena and Oscillatoria sp. Cyanophytes are thus recognized as potential toxic organisms and their blooms in drinking water or recreational reservoirs is of great importance.
Cyanobacterial toxins are contained within the living cells and are not released into surrounding water until senescence or death of the cells. Prechlorination during water treatment or the application of copper sulphate in controlling blooms (used in some European countries) results in cell death and release of toxins into water (Paerl 1988b, Carmichael and Falconer 1993). In 1988 within 2-3 weeks of copper treatment of the Goczalkowice reservoir, south Poland, the District Medical Officers recorded a rise in the number of cases of spastic bronchitis especially in children in this locality (Bucka and Zurek 1992). Although there have been no recorded cases of toxicosis in animals or humans in Ibadan, Omotosho (1985) reported some abnormalities in the gonads of Tilapia zillii and Oreochromis niloticus in the reservoir. In T. zillii, abnormal ovaries were unusually shaped with very thin membraneous ovarian wall and jelly-like ovarian tissue with few oocytes. However in O. niloticus unequal ovarian lobes and the occurrence of two pairs of ovaries were observed. It was suggested that massive development of eggs could be due to a breakdown in nervous and hormonal coordination in fish during the breeding period. Since both species of fish are algal feeders it is possible that the abnormalities observed could be related to their diet. It is therefore necessary to ascertain the presence or otherwise of toxins in cells of M. aeruginosa in Awba reservoir since both toxic and non-toxic blooms of the same species can be found in water. In view of the use of the reservoir for water supply, control measures aimed at preventing bloom formation such as reduction of nutrient load into the reservoir, artificial mixing of the water and biomanipulation of food-web components should be implemented.
Resumen
Las características físico-químicas y el fitoplancton de la represa Awba en la Universidad de Ibadan, Nigeria fueron monitoreadas para determinar el impacto de la eutroficación en la composición del fitoplancton. El análisis de componentes principales identificó tres denominados metales traza, oxígeno disuelto y composición iónica, como los mayores componentes que influencian la físico-química del agua. El análisis comparativo con un estudio previo mostró un fenómeno de incremento en los niveles de zinc, cobre e hierro en un periodo de 10 años. Además, la naturaleza ferruginosa del suelo contribuyó a los altos niveles de hierro que exceden los estándares de la Organización Mundial de la Salud para agua potable. La especie más abundante de fitoplancton fue Microcystis aeruginosa la que ha sido implicada en floraciones tóxicas en agua dulce. Se discuten las condiciones que favorecen el crecimiento de cianófitas y sus implicaciones.
References
Adebisi, A.A. 1981. The physico-chemical hydrology of a tropical seasonal river-Upper Ogun river. Hydrobiology 79: 157–165. [ Links ]
Adeniji, H.A. & I.G. Mbagwu 1990. Study of some physico- chemical factors and heavy metals in the Jakara reservoir, Kano state, Nigeria. Nat. Inst. Freshwat. Fish. Res. (NIFFR) Annual Report (1990): 136–140.
Anonymous 1984. Guidelines for drinking water quality, World Health Organization, pp. 17. In T.H.Y. Tebbutt (ed.). Principles of Water Quality Control, Oxford.
Anonymous 1985. Standard methods for the examination of water and wastewater. American Public Health Association (A.P.H.A.), Washington D.C. 1268 p.
Araoye, P.A. 2002. Man-made lakes, ecological studies and conservation need in Nigeria. Rev. Biol. Trop. 50: 857-864. [ Links ]
Aweto, A.O. 1994. Soil, pp. 49-57. In M.O. Filani, F.O. Akintola, C.O. Ikporukpo, (eds.). Ibadan Region. Rex Charles, Ibadan.
Barica, J. 1994. How to keep green algae in eutrophic lakes. Biology 49(4): 611–614. [ Links ]
Bidwell, A. & N.V. Clarke. 1977 The invertebrate fauna of Lake Kainji, Nigeria. Nig. Field 42 : 104–110. [ Links ]
Biney, C.A. 1990. A review of some characteristics of freshwater and coastal ecosystems in Ghana. Hydrobiology 208: 45–53.
Bryant, D.A. (ed.). 1994. The molecular biology of cyanobacteria. Kluwer. 881 p. [ Links ]
Bucka, H. 1989. Ecology of selected planktonic algae causing water blooms. Acta Hydrobiol. 31(3/4):207–258. [ Links ]
Bucka, H. & R. Zurek. 1992. Trophic relations between phyto- and zooplankton in a field experiment in the aspect of formation and decline of water blooms. Acta Hydrobiol. 34: 139–155. [ Links ]
Bucka, H. & E. Wilk-Wozniak. 1999. Cyanobacteria responsible for planktic water blooms in reservoirs in southern Poland. Algol. Stud. 94: 105–113. [ Links ]
Carmichael, W.W. &, I.R. Falconer. 1993. Diseases related to freshwater blue-green algal toxins, and control measures, pp. 187–209. In I.R. Falconer (ed.). Algal toxins in seafood and drinking water. Academic, London.
Carmouze, J.P., J.R. Durand & C. Leveque (eds.). 1983. Lake Chad: Ecology and Productivity of a shallow tropical ecosystem. Monogr. Biol. 53, The Hague.
Chapman, L.J. & Kramer, D.L 1991. Limnological observations of an intermittent tropical dry forest stream. Hydrobiol. 226: 153–166. [ Links ]
Ganf, G.G. 1974. Diurnal mixing and the vertical distribution of phytoplankton in a shallow equatorial lake (Lake George, Uganda). J. Ecol. 62: 611–629. [ Links ]
Green, J. 1972. Ecological studies on crater lakes in West Cameroon. Zooplankton of Barombi Mbo, Mboandong, Lake Kotto and Lake Soden. J. Zool. (Lond.) 166: 283–301. [ Links ]
Green, J. 1979. The fauna of Lake Sonfon, Sierra Leone. J. Zool., (Lond.) 187: 113-133. [ Links ]
Hare, L. & J.C.H. Carter. 1984. Diel and seasonal physico-chemical fluctuations in a small natural West African lake. Freshwat. Biol. 14: 597–610. [ Links ]
Holden, M.J. & J. Green. 1960. The hydrology and plankton of the River Sokoto. J. Anim. Ecol. 29: 65–84.
Kilhalm, P. & S.S. Kilhalm. 1980. The evolutionary ecology of phytoplankton, pp. 571–597. In I. Merris (ed.). The physiological ecology of phytoplankton. Blackwell.
Mombeshora, C., O. Osibanjo & S.O. Ajayi. 1981. Pollution studies on Nigerian rivers : Toxic heavy metal status of surface waters in Ibadan city. Envir. Int. 5: 49–53.
Mombeshora, C., O. Osibanjo & S.O. Ajayi. 1982. Pollution studies on Nigerian rivers: The onset of lead pollution of surface waters in Ibadan. Envir. Int. 9: 81–84. [ Links ]
Oduwole, G.A. 1990. Cumulative impact of effluents on dynamics of Awba dam. M.Sc Thesis, University of Ibadan, Nigeria. 86 p. [ Links ]
Omori, M. & T. Ikeda. 1984. Methods in marine zooplankton ecology. Wiley, 330 p. [ Links ]
Omotosho, J.S. 1985. Gonad structure embryonic and larval development in two cichlids – Tilapia zilli (Gervais) and Sarotherodon niloticus Linnaeus (Trewavas) Ph.D. Thesis University of Ibadan, Nigeria.
Paerl, H.W. 1988b. Nuisance phytoplankton blooms in coastal, estuarine and inland waters. Limnol. and Oceanogr. 33: 823–847. [ Links ]
Paerl, H.W. & C.S. Tucker. 1995. Ecology of blue-green algae in aquaculture ponds J. Wld. Aquacult. Soc. 26(2): 109 –131. [ Links ]
Payne, A.I. 1986. The ecology of tropical lakes and rivers. Wiley, 301 p. [ Links ]
Raymont, J.E.G. 1980. Plankton and Productivity in the Oceans. Volume 1: Phytoplankton. Oxford. pp. 302 & 324.
Reynolds, C.S. & D.A. Rogers. 1976. Seasonal variations in the vertical distribution and buoyancy of Microcystis aeruginosa Kutz. Emend. Elenkin in Rostherne mere, England. Hydrobiol. 48: 17–23.
Reuter, J.G. & R.R. Petersen. 1987. Micronutrient effects on cyanobacterial growth and physiology. New Zeal. J. Mar. Freshwat. Res. 21: 435–445. [ Links ]
Reynolds, C.S., G.H.M. Jaworski, H.A. Cmiech & G.F. Leedale. 1981. On the annual cycle of the blue-green alga Microcystis aeruginosa Kutz. Emend. Proc. R. Soc. Lond. B. Biol. Sci., 293: 419-477.
Reynolds, C.S. 1984a. The ecology of freshwater phyto-plankton. Cambridge. 384 p. [ Links ]
Reynolds, C.S. 1984b. Phytoplankton periodicity: The interaction of form, function and environmental variability. Freshwat. Biol., 14: 111–142. [ Links ]
Reynolds, C. S. 1998. What factors influence the species composition of phytoplankton in lakes of different trophic status? Hydrobiology 369/370: 11–26. [ Links ]
Segers, N., C.S. Nwadiaro & H.J. Dumont. 1992. Rotifera of some lakes in the floodplain of the River Niger (Imo state, Nigeria) II. Faunal composition and diversity Hydrobiology 250: 63–71. [ Links ]
Sommer, U. 1981. The role of r- and k- selection in the succession of phytoplankton in lake Constance. Acta Oecol. 2: 327–342. [ Links ]
Trollope, D.R. & B. Evans. 1976. Concentrations of Copper, Iron, Lead, Nickel and Zinc in freshwater algal blooms. Envir. Pollut. 11: 109–116.
Ugwumba, A.A.A. & A. Adebisi. 1992. The food and feeding ecology of Sarotherodon melanotheron (Ruppell) in a small freshwater reservoir in Ibadan, Nigeria. Arch. Hydrobiol. 124 (3): 367–382.
Van de Velde, I. 1978. Cladocera and Copepoda from the valley of the river Senegal. Biol. Jb. Dodonala 46: 192-201. [ Links ]
Wright, R. 1982. Seasonal variations in water quality of a West African river (R. Jong in Sierra Leone) Rev. Hydrobiol. Trop. 15: 193–199. [ Links ]
1 Present Address: School of Life Sciences, Robert Gordon University, St. Andrews Street, Aberdeen AB 25 1HG; gakinoriola@yahoo.com; ext.akin-oriola@rgu.ac.uk














