Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.51 n.1 San José Mar. 2003
Currently biological invasions are considered one of the worlds most serious conservation problems. Ligustrum lucidum is the most abundant exotic tree in secondary forest patches of montane forests of NW Argentina. We studied the determinants of success of the early stages of its life cycle in distinct habitat types, with the hope of identifying vulnerabilities that could be exploited to control the invasion. Seed arrival, germination, seedling recruitment and survival, and sapling growth were studied in edges, gaps and forest interior. Seed arrival was also assessed under perches and in open fields. Germination was studied in forest and grass-land patches. L. lucidum seedling survival and sapling growth were compared with the most abundant native species survival and growth. Seed arrival was strongly seasonal with a peak in mid-August. Seed rain did not differ significantly among habitat types, however there was a tendency for edges to receive more seeds when only dispersed seeds were considered. Perches strongly enhanced seed arrival; more than 40 times the number of seeds were dispersed beneath citrus plants (i.e. perches) than found in paired open areas. In the forest, seeds in gaps and edges had higher germination rates, but there was no difference in seedling survival. Fruits under closed canopy exhibited the lowest germination. Germination and survival were low in open areas. Neither seedling recruitment nor sapling growth differed between gaps and forest interior. L. lucidum saplings grew significantly more than saplings of the most common native species, and also showed higher seedling survival. L. lucidum is a prolific fruit producer, is capable of germinating and surviving in a broad range of forest environments, it is relatively shade tolerant and has higher survival and faster growth rate in comprison to the most common native species. All these characteristics highlight its potency as a successful invader, and point to few vulnerabilities that could be targets of control measures.
Key words: Biological invasions, exotic species, secondary forest, montane forest, gaps, forest edges, Ligustrum lucidum
Invasive species and their capacity to transform natural ecosystems have long caught the attention of ecologists (e.g., Elton 1958). Although the invasion process is not in itself unnatural, the rate at which species reach new habitats has clearly increased during the 20 th century (Lodge 1993). The movements of organisms beyond their natural range can have several consequences, from slightly changing the species composition to causing species extinction or affecting ecosystem function (Vitousek and Walker 1989, Kareiva 1996, Parker and Reichard 1997). Ecologists have tried to identify characteristics which are common to invasive species (Baker 1974, Crawley 1986, Binggeli 1996) mainly with the hope of finding rules that may improve our predictive ability, and ultimately guide us towards practices that would reduce the risk of invasions. In the face of this current concern, studies about life history characteristics of invaders and how they vary in different habitats have gained importance for theoretical and practical purposes (D Antonio 1993, Rejmánek and Richardson 1996).
Many invasive species show high seed production, effective dispersal, no special environmental requirements for germination or flowering, rapid seedling growth and high interspecific competitive ability (Baker 1974, Rejmánek and Richardson 1996, Vilá and DAntonio 1998). In general, early life history characteristics appear to play an important role in determining the outcome of an invasion (Harper 1977, Drake et al. 1989). In addition, invasive species are also often associated with disturbances (Hobbs 1989, D Antonio and Vitousek 1992). Canopy gaps and edges represent common disturbances in forest ecosystems. Canopy gaps influence germination, seedling recruitment, sapling growth, flowering and fruiting patterns (Collins and Pickett 1988, Denslow et al. 1990, Pacheco and Grau 1997, Van der Meer et al. 1998) and represent opportunities for colonizing species to reach and establish in a mature community (Brokaw 1985, Grau 1999). Edges constitute a substantial portion of forest environments (Chen and Frankling 1992, William-Linera 1993), especially in secondary patches that are often isolated in a matrix of agricultural fields or other developed areas. The success of an exotic species can be tied to its ability to colonize these different habitat types.
Several protected areas in Argentina are beset with infestations of exotic species making exotic control a critical management priority (Di Pietri 1992, Grau and Aragón 2000). More specifically, in the protected areas of the NW (mainly neotropical forests) there are several records of the presence of exotics but no information about these species is circulated among the general public or protected area managers (Grau and Aragón 2000). Understanding more about the biology of potential invaders and their habitat requirements represents a first step in designing management strategies by allowing us to determine what issues are likely to act as obstacles to conservation.
In this paper, we examine the invasion of secondary forest patches in NW Argentina by an exotic tree, Ligustrum lucidum (W. T. Aiton, 1810) (Oleaceae). L. lucidum is an evergreen tree native to Asia that is considered invasive in Australia, New Zealand and riparian forests of SE Argentina (Montaldo 1993, Cronk and Fuller 1995). Ligustrum robustum (Blume, 1850) and Ligustrum sinense (Lour., 1790) are cited as highly invasive in other subtropical and tropical areas (Cronk and Fuller 1995). Secondary forest patches are an important component of the Neotropical Mountain forest of NW Argentina, and they comprise the boundaries of many protected areas (Grau et al.1997).
The aim of this study was to describe L. lucidum performance in early stages of its life cycle in different habitats. Our main questions were: (1) Is there any particular stage in which L. lucidum is especially vulnerable?, (2) Is L. lucidum success equal in different habitat types?, and (3) What is the relative performance of L. lucidum and native species?. The underlying goal of this work was to identify some vulnerabilities that may be useful in controlling the invasion by L. lucidum.
Study area: The study was conducted in the lower montane forest of Sierra de San Javier 27º30 S, 65º40 W), Tucumán, Argentina. The vegetation corresponds to the phytogeographic province of the Argentinean "Yungas" (Cabrera 1976) and is considered the sourthern end of the Neotropical Montane forests along the eastern slope of the Andes. The study sites were located within the boundaries of Parque Biológico Sierra de San Javier (PBSSJ), a protected area of 14 000 ha located 15 km west of San Miguel de Tucumán, the capital of the province. Mean annual temperature is 18 o C, and average rainfall ranges from 969 to 1 448 mm with most precipitation falling during the summer (Hunzinger 1997).
The project was carried out in secondary forest patches between 600 to 700 m, close to the boundaries of PBSSJ. The vast majority of this area was deforested during the earlymid 1900s, and planted with citrus orchards and annual crops. After the creation of PBSSJ these lands were abandoned and are now in different stages of recovery (Grau et al. 1997).
Materials and methods
We used an observational and an experimental approach to study the characteristics of L. lucidum in different stages of its life cycle in different habitats. Seed arrival, germination and seedling survival were studied in edges, canopy gaps and forest interior. Seed arrival was also assessed under perches (i.e., citrus plants) and in open areas. In addition, we created canopy gaps by cutting down adult L. lucidum trees and naturally occurring seedling recruitment and sapling growth were studied in these gaps and under intact canopy.
Seed arrival
To examine temporal and spatial patterns of seed rain, we used fruit traps placed in each habitat type. Traps were checked biweekly from June to September 1998, and on each occasion, seeds were counted and classified as either Dispersed Seeds or Fallen Fruits. L. lucidum dispersed seeds are easily distinguished from fallen fruits because birds digest the exocarp and part of the pulp. In addition, dispersed seeds are often found in clumps. We considered both fallen fruits and dispersed seeds because even though germination success from intact fruits is low (Burrows and Kohen 1986, R. Aragón, unpublished), given the masive fruit production of L. lucidum, fallen fruits may account for a significant number of established seedlings. Bird consumption is important because it potentially affects germination (i.e. removing of the exocarp) (Burrows and Kohen 1986) and allows dispersal to new sites.
We used a randomized complete block design with forest interior, edges and gaps as treatments. A block (i.e. replicate) consisted of three transects, one transect in each habitat type (i.e. forest interior, edges and gaps) and with four traps per transect as subsamples. The transects within each block were no more than 10 m apart. We worked with 10 replicates (i.e. blocks) and a total of 120 traps (10 blocks x 3 treatments x 4 traps). Seed traps (0.50 x 0.50 m plastic mesh plots) in each transect were approximately 3 m apart from each other. Seeds gathered in the four traps per transect were summed. To avoid the bias that could occur if distance to seed sources affected the treatments differentially we took two precautions. We placed all our replicates in forest patches where L. lucidum was very abundant (approximately 300 individuals per ha) and we used a block design (i.e. transects that represented each treatment within each block were separated for a maximum of 10 m).
To assess the role of perches in seed arrival, we worked in an abandoned citrus orchard distinguishing two treatments: open areas and under citrus plants (i.e., perches). Traps were placed in a paired design beneath citrus plants and in nearby open areas (not more than 5 m apart). Trap area was 1 m 2 with 10 replicates per treatment. Traps were checked weekly from June to September 1998 and the number of dispersed seeds was counted. Given that traps under citrus plants and in open areas within each replicate were placed only 5 m apart, we considered that distance to seed sources could seldom affect seed arrival in the two treatments differently.
Analysis: The seasonal pattern of seed arrival in each habitat type was analyzed through a repeated measures ANOVA. Seed counts were considered repeated measurements of habitats and the difference among treatments (i.e., habitat types) was tested as the linear and quadratic terms of the interaction between treatments and time (i.e., testing if the treatments had the same slopes). In these analyses we considered first the total seed arrival (the sum of fallen fruits and dispersed seeds) and then, only the dispersed seeds.
The overall seed arrival to each habitat type was compared by performing a two-way ANOVA by permutations. The randomization version of ANOVA involves calculating an F statistic from the observed data and recording what percentage of times this is exceeded by values obtained from randomized data (Manly 1997). P- values were determined based on 10 000 permutations. Due to variance heterogeneity, a two-sample t-test for unequal variances was used for the comparisons among citrus and open areas. In both cases, we used square root transformed seed counts.
Germination and survival
L. lucidum germination and survival was assessed by a field experiment in an old growth forest patch. We considered the same three situations as in the seed rain section above: forest interior, edges and gaps, with five replicates of each. In June 1998, L. lucidum fruits were collected directly from trees and were peeled manually. They were kept at room temperature for no more than a week until the trial started. In each of the five replicates, 100 seeds were placed directly on the soil in two groups of 50 seeds surrounded by a wire mesh to avoid wash out. In the forest interior, we included a treatment of fallen fruits (without removing the exocarp) in addition to the seeds, summing a total of four groups in each replicate of this treatment.
Germination and survival were recorded every 7 to 10 days from July 1998 to January 1999 and seedlings were marked as they emerged. Germination was defined as the first sign of the white root tip visible outside the endocarp. Survival of seedlings was monitored for six months. Here, survival was computed as the ratio of the number of seedlings present in a given plot at the end of the experiment and the number of seeds that germinated in that plot.
At the same time and in a similar fashion, we performed germination trials in open fields or grasslands. In each of the 8 replicates, we set up four plots with 50 seeds each, and in two treatments (two plots per treatment): areas of bare ground (i.e., "disturbated" areas where we periodically removed litter and ground vegetation) and areas with intact cover. Seeds that germinated were recorded every 7-10 days from July through December 1998.
Analysis: Randomization tests were used to compare germination and survival among treatments within forest (two-way ANOVA by permutations) or open field trials (two sample randomization test). In both cases, homogeneity of variance was checked. Due to an early lost of replicates in the grassland sites we were able to compute survival based on only three replicates and no statistical test was performed to compare forest vs. open field trials.
Change in canopy cover: An experimental approach
Higher light environments have been cited as a primary cause of changes in seedlings and saplings growth rate in forest environments (Brokaw 1985, Van der Meer et al. 1998). To see how canopy openings affect L. lucidum survival and growth, we created canopy gaps by cutting adult L. lucidum trees. Gaps sizes were similar to the naturally occurring gaps in secondary forest patches of this area (i.e., between 10 to 20 m in their longest axis) (Grau 1999).
Saplings and trees were tagged and measured at the beginning of the experiment and one year later in five gaps and five 10 x 10 m paired plots under intact canopy cover. Diameter at breast height (DBH) was measured in all woody saplings and trees taller than 2 m. Diameter at 5 cm from the base was measured in saplings taller than 0.10 m and smaller than 2 m. We also established two 1 x 1 m subplots in every gap and closed-canopy plot where we recorded spontaneous seedling recruitment monthly over one year. Total recruitment was defined as the number of individuals of each species recorded over one year period. Survival was computed in the same way as in Germination and survival above.
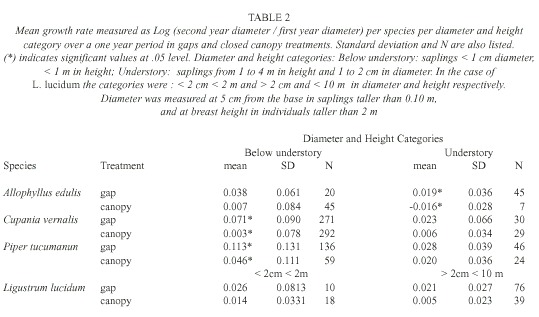
Analysis: We compared L. lucidum saplings with the three most abundant native species: Allophyllus edulis (Radfk., 1890), Cupania vernalis (Cambess., 1825) and Piper tucumanum (C.DC., 1898) and saplings were assigned to three diameter-height categories. These categories were chosen based on the main strata in these forests. Below-understory saplings (< 1 m in height, < 1 cm diameter) were found under the Psychotria carthagenensis (Jacq., 1790) stratum, which is the main species that forms the understory. Saplings that were 1 to 4 m in height were part of the under-story (between 1 and 2 cm in diameter), and saplings (from 2 to < 10 cm in diameter) and trees taller than 4 m constituted the subcanopy and canopy strata. In the case of L. lucidum , the two first diameter classes were pooled for the analysis.
Differences between treatments were tested using an Analysis of Covariance (ANCOVA) with the log of the ratio [(diameter of the second year)/ (diameter of the first year)] as a response variable and log (diameter of the first year) as a covariate. In all cases, residual normality was checked. Using also the log of the ratio of the two diameters as a response variable we tested for differences between species through a splitplot analysis with treatment (gaps and close canopy plots) as the whole plot factor and species (A. edulis, C. vernalis, P. tucumanun and L. lucidum) as the subplot factor.
We compared the recruitment of the most abundant species in gaps and under closed canopy using a two-sample randomization test (Manly 1997), checking first for equality of variances. After 5 000 permutations P-values were determined as the percentage of random differences that were equal or larger than the observed difference. The total number of seedlings that were recorded per replicate over one year was used as the response variable. Only the species that had more than 10 records were included in the analysis (C. vernalis, P. tucumanun, L. lucidum, L. sinense, P. carthagenensis, and Myrsine laetevirens). First, we compared the total number of seedlings in gaps and closed canopy for these six species combined, and then we considered each species separately.
Seedling survival was measured as the ratio between the number of seedlings that were present at the end of the experiment and the total number of seedlings that emerged. We performed a two-sample randomization test as with recruitment above. Analyses were done with SAS 6.12 and RT- 2.0.
Results
Seed arrival
L. lucidum seed rain was strongly seasonal with a peak in mid-August (Fig. 1a, date 5). Averaging over time, there was no difference in seed arrival (defined as the number of fallen fruits plus the number of dispersed seeds) among treatments (F= 2.45, p=0.11, d.f.= 2, 18). Only the quadratic component showed a statistically significant relationship between seed arrival and time (F= 19.52, p=0.0003, d.f.= 1, 18). Averaging over treatments, there was a significant difference between biweekly intervals considered (F=6.15, p=0.005, d.f.= 2, 6), with the maximum seed arrival in August. The total seed arrival (i.e., the sum of the seeds collected in each habitat type throughout the fruiting season) did not differ significantly among habitat types either (p=0.20, ANOVA by permutations), but showed a significant difference among replicates (i.e., blocks) (p=0.01, ANOVA by permutations). Some traps consistently received more seeds throughout the entire fruiting period.
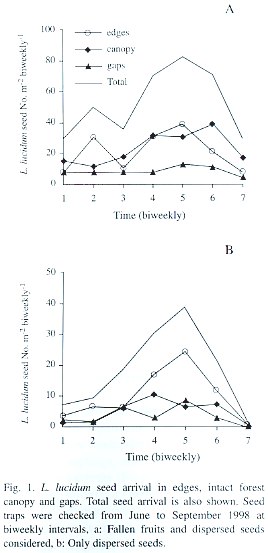
Seeds that showed signs of having been carried by birds were taken as a measure of dispersal to each habitat type. The overall quantity of dispersed seeds (defined as the average number of dispersed seeds collected per date in the three habitat types) was mainly driven by seed arrival to edges, and also showed a peak in mid-August (Fig. 1b, date 5). The average seed arrival changed over the fruiting season (F= 3.10, p=0.007, d.f.= 6, 108; Table 1). During mid-August an average of 24 seeds were recorded in each replicate (1 m2 ) in edges, in comparison to 6.3 and 8.4 seeds under close canopy and gaps respectively. The quadratic component of the seed dispersal response curve was significantly different from 0 (Time [quadratic] F=11.31 p= 0.003, d.f.= 9, 18; Table 1), but the pattern of seed dispersal did not differ among habitat types (F=0.27 p=0.76, d.f.= 2, 18, for the interaction between the linear component and treatment and F=1.92 p=0.17, d.f.= 2, 18, for quadratic* treatment interaction; Table 1).
When considering the overall seed dispersal (i.e., the total number of seeds collected in each habitat type throughout the entire fruiting season) edges showed a slight tendency to receive more seeds in comparison to gaps and closed canopy habitats (p=0.08, ANOVA by permutations). As when fruits were considered, there was a large variation between replicates (p=0.007 ANOVA by permutations).
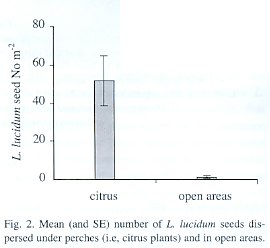
More than 40 times the number of seeds were dispersed beneath citrus plants than were found in paired open areas (t=3.84 p=0.003) (Fig. 2). This difference between treatments was maintained through the fruiting season and seed arrival showed a peak in the last week of July.
Germination and survival
In both the forest sites and the open fields, seeds placed in the plots did not start to germinate until September. Five of the open field plots were vandalized during the course of the experiment, and although no statistical test was performed, germination of L. lucidum seeds seemed lower in open fields than in the forest sites (13 and 43% respectively). The germination that did occur in open fields showed no difference between areas with bare ground and areas with intact cover (p=0.24, permutation test). Many seeds in the open field experiment were removed or damaged as soon as they were placed in the field. Many of them also showed signs of desiccation, and there were no seeds remaining after the trial ended.
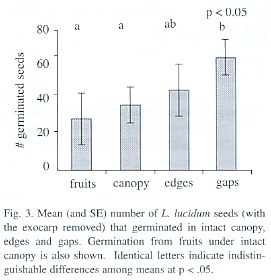
Percentage of germination in the forest trial was highly variable in different replicates and treatments, ranging from 8 to 75%. Germination differed among treatments (p= 0.05, permutation test), with gaps and edges having the greatest values. Fruits under closed canopy exhibited the lowest germination (Fig. 3). The survival of seedlings was similar between the different treatments with a slight tendency for gaps to have lower survival (p=0.08, permutation test). Mean seedling survival per treatment (i.e., averaging over the replicates) ranged from 60 to 98%.
As stated above many grassland (i.e., open fields) plots were lost during the course of the experiment and survival data were taken only from three plots. In these plots only 10 to 20% of the seedlings survived until the end of the trial (January 1999). Although no statistical test was performed, percentage of survival in these few plots was lower in grasslands than in the forest trial.
Change in canopy cover
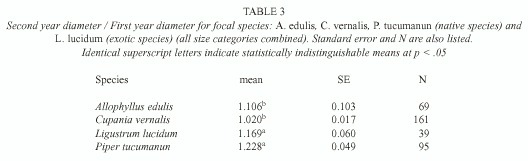
Sapling growth: Only in three cases saplings grew significantly more in gaps: C. vernalis below understory saplings, A. edulis understory saplings, and P. tucumanun below understory saplings (F= 4.80, p=0.03, d.f.= 1, 7, F= 4.94, p=0.05, d.f .= 1, 3, F=5.67, p= 0.02, d.f.= 1, 22, respectively) (Table 2). Treatments had no effect in any of the L. lucidum diameter-height categories (F=0.01, p=0.97, d.f.= 1, 1 for the below understory/understory category and F= 2.71, p=0.15, d.f.=1, 6 for the above understory category) (Table 2). Change in diameter over one year was different among the species (F for species [subplot factor]= 4.77, p=0.006). L. lucidum and P. tucumanum had higher diameter growth rates in comparison to A. edulis and C. vernalis (Table 3).
Seedling recruitment and survival: The total number of seedlings that naturally emerged over one year, did not differ between gaps and under closed canopy. However, there was a marginal tendency towards finding more seedlings in gaps (p=0.08, permutation test).
There was no difference in seedling recruitment when considering each species separately, only P. carthagenensis, showed a slight tendency to have more seedlings in gaps (p=0.11, permutation test). L. lucidum seedlings recruited equally in gaps and under closed canopy (p=0.43, permutation test). Survival of all the six focal species combined did not differ between treatments (p=0.34, permutation test). L. lucidum was the only species that showed higher seedling survival in gaps (p=0.03, permutation test). Average survival of L. lucidum seedlings appeared to be higher than the average for the rest of the species (0.874 [canopy] and 0.940 [gaps] for L. lucidum vs. 0.543 [canopy] and 0.522 [gaps] for the remaining five species combined, respectively).
Discussion
Many aspects of the biology of L. lucidum make it a good invader in these secondary forest patches. L. lucidum is capable of germinating and surviving in a broad range of forest habitats. Its seedlings and saplings can grow satisfactorily regardless of light conditions. It also showed higher survival and growth rate in comparison to some of the most common species in this area. Like other successful exotic woody species it is bird dispersed (Binggeli 1996), and is consumed by the most abundant birds in the area (Aragón 2000). In addition, it is able to germinate from both fallen fruits and dispersed seeds. Although germination from fallen fruits is low, given its profuse seed production, fallen fruits may play an important role in L. lucidum permanence in a given patch. Its frequent consumption by birds and its ability to germinate from intact fruits high-light L. lucidum capacity of recruitment in new or already colonized patches. All these attributes reveal its potential as a invader, already successful in this area and certainly dangerous in similar communities.
Many studies found that seed rain is more abundant in forest edges and gaps (e.g. Blake and Hoppes 1986, Herrera et al. 1994), mainly as a result of the foraging behavior of the dispersers. Although we found no statistical difference in seed arrival among habitat types, there was a tendency for edges to receive more seeds when only dispersed seeds were considered. The most abundant local dispersers in this area (Turdus rufiventris and Thraupis sayaca) are generalized frugivores (R. Aragón, unpublished), and although data about their pattern of habitat use are only anecdotal, we would expect them to use forest edges frequently. At the same time, we expect that these species foraging patterns would be highly influenced by plant cover, and more precisely, by the presence of perches. Seed shadow generated by avian dispersers is context-dependent (Herrera et al. 1994), and even in sites a couple of meters apart, the vertical structure (cover and composition) can be quite variable and this can affect seed shadow. Two of the three replicates that consistently received more seeds were close together and shared similar vegetation structure. Despite all our replicates were located in patches where L. lucidum was dominant, it is possible that slight changes in plant cover or the presence of L. lucidum or other species fruiting trees (e.g. P. carthagenensi s) close to a particular seed trap, could have influenced foraging behavior, and therefore, seed shadow. All these factors can affect seed arrival and determine how many seeds are dropped in a given square meter (Stiles 1980, Sargent 1990, Pacheco and Grau 1997). This highlights the importance of seed dispersal in determining the spatial pattern of seed rain. Due to this high variability in seed rain (dispersed seeds) even within the same habitat type is possible that the sampling effort was insufficient to detect differences across habitats.
Perches play a significant role in bird dispersed species (McDonnel and Stiles 1983, Read and Hill 1983, Sallabanks 1992). Not only plants, but also fences and posts, can serve as perches for birds, and in turn can affect the pattern of seed rain (Read and Hill 1983). In this case, seed arrival was much more frequent under citrus plants than in open areas. Before the creation of this protected area, citrus plantations were one of the most common land uses in the piedmont of Sierra de San Javier. When citrus orchards were abandoned, land owners simply stopped cleaning the area, but did not actively remove their fruit trees unless they planted it with another crop. Many patches that are now colonized by L. lucidum were citrus orchards (Grau et al. 1997, Grau and Aragón 2000). The spatial distribution of seedlings that are clumped around old citrus plants or stumps in abandoned orchards is consistent with dispersal occurring primarily underneath perches (McDonnel and Stiles 1983, Sallabanks 1992). The scarcity of perches may delay colonization by L. lucidum until the first shrubs and trees get established.
In addition to limits imposed by poor seed dispersal, the establishment of L. lucidum in open fields is constrained by low germination success and high seedling mortality. Grasslands are a more hostile environment due to enhanced seed predation (mainly by rodents and birds), higher evapotranspiration rates, and greater variability in both temperature and moisture conditions (Uhl et al. 1988, Manson and Stiles 1998). Colonization by Myrica faya in open sites in Hawaii is reduced due to its inability to germinate and survive, as well as by the lack of seed dispersal to this kind of habitat (Vitousek and Walker 1989). This may be also the case in the first stages of colonization of open fields by L. lucidum. However, the invasibility of open sites may change after a few shrubs and trees that attract dispersers and provide protection have established.
Tree fall gaps seem to provide good intermediate conditions in terms of moisture and light for seed germination; however this did not have a clear effect on L. lucidum seedling establishment and survival. Seed dispersal into gaps may be subject to enhanced seed predation and seedling mortality, which could balance the effect of an increase in growth and sapling survival (Schupp 1988). In the case of L. lucidum a trend was clear for higher seed germination in gaps but not for seedling survival. Seedling survival was lower in gaps in our six-month trial (i.e. survival experiment in natural gaps), but higher when considering the results of the one-year experiment. This seems to indicate that seedling mortality (mainly due to desiccation and herbivory) may be higher in gaps in the early stages of establishment, but after seedlings survive the dry season (first months of both experiments), overall survival is higher in gaps. Although no measurements were taken, seedlings in gaps seemed larger than seedlings under close canopy.
Saplings grew equally well in a wide range of light availability from closed canopy sites to edges. This makes L. lucidum less dependent on disturbances such as tree fall gaps. L. lucidum saplings are abundant under its own canopy, and given that it is an evergreen tree, the understory is quite shaded all year. In contrast, some subcanopy species (i.e., C. vernalis and A. edulis) may depend on gaps for their survival and growth. Sapling growth rate of native canopy species and L. lucidum could not be compared due to the small sample of canopy species. L. lucidum saplings have a diameter growth rate similar to P. tucumanun, an understory small tree. Other species of the genus Piper have been cited as fast growing species that respond to gaps (Marquis 1988, Denslow et al. 1990). L. lucidum growth rate is higher than rates of the subcanopy natives, C. vernalis and A. edulis. This may also represent an advantage, especially in low light conditions existing within L. lucidum patches.
In summary, this detailed analysis reveals many attributes that can explain the success of L. lucidum as an invader. L. lucidum has a prolific fruit production, it is consumed by the most common species of birds in the area (Aragón 2000). L. lucidum establishes and survives in a wide range of conditions, although it appears to be a poor colonizer of the first stages of succession in open habitats. It may recruit better into areas with some canopy cover, both because its principle dispersers prefer areas with perches, and because the conditions for germination are more favorable. L. lucidum is relatively shade tolerant and has a fast growth rate even in the dark L. lucidum understory. In addition, L. lucidum grows more rapidly than most of the native species, and thus may outcompete natives once it has become established in a plot.
Given the many advantages to L. lucidum as an invader, control of this invasion may only be possible at early stages in the invasion process. The control of invasive species is usually economically restrictive and ecologically infeasible when they are already widespread in an area (Simberloff 1997). The eradication of L. lucidum from patches when it is already dominant is extremely difficult (Little 1982), however control of its establishment in new patches may be feasible. The highest priority should be given to discouraging its cultivation as landscape plants in houses or roadsides to avoid colonization of new areas and actively eradicating any trees that could serve as seed sources. Unfortunately, these results point to few vulnerabilities that could be targets of control measures.
Acknowledgements
This research was supported by CONICET and Sigma Xi fellowships. We are indebted to P. Vaquera, K. Buzza and A. Novillo for field assistance. D. Dickey, J. Gilliam, C. Brownie, T. Wentworth and R. Grau all provided critical comments on the study design, analysis and early versions of the manuscript.
Resumen
Las invasiones biológicas son consideradas actualmente uno de los problemas de conservación más serios. Ligustrum lucidum es el árbol exótico mas abundante en los bosques secundarios del NO de Argentina. Estudiamos llegada de semillas, germinación, reclutamiento y crecimiento de renovales en claros, bordes e interior del bosque La llegada de semillas también se estudió bajo perchas y en sitios abiertos. La supervivencia y el crecimiento de renovales de L. lucidum fueron comparados con los de las especies nativas mas abundantes. El arribo de semillas fue estacional y los bordes mostraron una tendencia a recibir mas semillas dispersadas. La presencia de perchas aumentó la llegada de semillas. Dentro del bosque, las semillas germinaron mas en claros y bordes, pero no hubo diferencia en sobrevivencia. La germinación y la supervivencia fue baja en sitios abiertos. El reclutamiento y crecimiento de renovales no difirió entre claros e interior del bosque. Los renovales de L. lucidum crecieron mas que los renovales de las especies nativas mas comunes. L. lucidum se establece y sobrevive en un amplio rango de condiciones, es relativamente tolerante a la sombra y tiene una rápida tasa de crecimiento. Estas características indican su capacidad invasora, y señalan escasas vulnerabilidades que puedan ser usadas para su control.
References
Aragón, R., 2000. Especies exóticas como recurso para las aves, pp. 21- 35. In H.R. Grau & R. Aragón (eds.). Arboles Exóticos de las Yungas Argentinas. LIEYUNT. Argentina.
Baker, H.G., 1974. The evolution of weeds. Ann. Rev. Ecol. Syst. 5: 1-24. [ Links ]
Binggeli, P. 1996. A taxonomic, biogeographical and ecological overview of invasive woody plants. J. Veg. Sci. 7: 121-124. [ Links ]
Blake, J.G. & W.G. Hoppes. 1986. Influence of resource abundance on use of tree-fall gaps by birds in an isolated woodlot. The Auk 103: 328-340. [ Links ]
Brokaw, N.L.V. 1985. Treefalls, regrowth and commnunity structure un tropical forest. In S.T.A. Pickett & P.S. White (eds.). The Ecology of Natural Disturbances and patch dynamics. Academic, NY. USA.
Burrows, F.J. & J. Kohen. 1986. Inhibition of germination in privet. Plant Prot. Quartely 1: 107-108. [ Links ]
Cabrera, A. 1976. Regiones Fitogeográficas Argentinas. ACME. Buenos Aires. Argentina. [ Links ]
Chen, J. & J.F. Frankling. 1992. Vegetation responses to edge environments in old growth Douglas fir forest. Ecol. Appl. 2: 387-396. [ Links ]
Crawley, M. 1986. The Population biology of invaders. Phil. Trans. R. Soc. Lond. 314: 711-731. [ Links ]
Collins, B.S. & S.T.A. Pickett. 1988. Demographic responses of herb layer species to experimental canopy gaps in a northern hardwoods forest. J. Ecol. 76: 437-450.
Cronk, Q. & J. Fuller. 1995. Plant Invaders: The threat to natural ecosystems. Chapman and Hall. London. UK. 241 p. [ Links ]
DAntonio, C. 1993. Mechanism controlling invasion of coastal plant communities by alien succulent Carpobrotus edulis. Ecology 74: 83-95. [ Links ]
DAntonio, C. & P. Vitousek. 1992. Biological invasions by exotics grasses, the grass/fire cycle, and global change. Ann. Rev. Ecol. Syst. 23: 63-87. [ Links ]
Denslow, J.A., J.C. Schultz, P.M. Vitousek & B.R. Strain. 1990. Growth responses of tropical shrubs to treefall gap environments. Ecology 71: 165-179. [ Links ]
De Pietri, D.E. 1992. Alien Shrubs in a national park: can they help in the recovery of natural degraded forest?. Biol. Conserv. 62: 127-130. [ Links ]
Drake, J., F. di Castri, R. Groves, F. Kruger, H. Mooney, M. Rejmanek & M. Williamsom. 1989. Ecological Invasions: a global perspective. Wiley, Chichester, England. 525 p.
Elton, C.S.1958. The ecology of invasions by animals and plants. Methuen, London, UK. 182 p. Grau, H.R. 1999. Disturbances and tree species diversity along the elevational gradient of subtropical montane forest of NW Argentina. Ph. D. Thesis, University of Colorado at Boulder, Colorado, US. 173 p.
Grau, H.R., M.F. Arturi, A.D. Brown & P.G. Aceñolaza. 1997. Floristic and structural patterns along a chronosequence of secondary forest succession in Argentinean subtropical montane forest. For. Ecol. Manage. 95: 161-171.
Grau, H.R. & R. Aragón. 2000. Ecología de los árboles invasores de la Sierra de San Javier, p. 5- 20. In H.R. Grau & R. Aragón (eds.). Arboles Exóticos de las Yungas Argentinas. LIEY-UNT. Argentina. [ Links ]
Harper, J.L. 1977. Population biology of plants. Academic, London, England. 892 p. [ Links ]
Herrera, C.M., P. Jordano, L. Lopez Soria & J.A. Amat. 1994. Recruitment of a mast-fruiting, bird dispersed tree: bridging frugivore activity and seedling establishment. Ecol. Monogr. 64: 315-344.
Hobbs, R.J. 1989. The nature and effects of disturbance relative to invasions, pp. 389- 405. In J. Drake, F. di Castri, R. Groves, F. Kruger, H. Mooney, M. Rejmanek & M. Williamsom (eds.). Ecological Invasions: a global perspective. Wiley, Chichester, England.
Hunzinger, H. 1997. Hydrology on montane forest in the Sierra de San Javier, Tucumán, Argentina. Mountain Research and Development 17: 229-308. [ Links ]
Kareiva, P. 1996. Developing a predictive ecology for Non-indigenous species and ecological invasions. Ecology 77: 1651-1652. [ Links ]
Little, C. 1982. How to control privet. NZ J. of Agriculture 1: 15. [ Links ]
Lodge, D.M., 1993. Biological Invasions: Lessons for Ecology. Trends Ecol. Evol. 8: 133-137. [ Links ]
Manly, B.F.J. 1997. Randomization and Monte Carlo methods in biology. Chapman and Hall. New York. USA. 399 p. [ Links ]
Manson, R.H. & E.W. Stiles. 1998. Links between micro-habitat preferences and seed predation by small mammals in old fields. Oikos 82: 37-50. [ Links ]
Marquis, R.J. 1988. Phenological variation in the Neotropical understory shrub Piper arielanun: causes and consequences. Ecology 69: 1552-1565. [ Links ]
Mc Donnel, M.J. & E.W. Stiles. 1983. The structural complexity of old-field vegetation and the recruitment of bird-dispersed plant species. Oecologia 56:109-116. [ Links ]
Montaldo, N.H. 1993. Dispersión por aves y éxito reproductivo de dos especies de Ligustrum (Oleaceae) en un relicto de selva subtropical en Argentina. Rev. Chil. Hist. Nat. 66: 75-85.
Pacheco, S. & H.R. Grau. 1997. Fenología de un arbusto del sotobosque y ornitocoria en relación a claros en una selva subtropical de montaña del Noroeste Argentino. Ecología Austral 7: 35-41. [ Links ]
Parker, I.M. & S.H. Reichard. 1997. Critical Issues in Invasion Biology for Conservation Science, pp. 283-305. In P.L. Fiedler & P.M. Kareiva (eds.). Conservation Biology for the Coming Decade. Chapman and Hall. New York. USA.
Read, J. & R.S. Hill. 1983. Rainforest invasion onto Tasmanian old-fields. Aust. J. Ecol. 8: 149-161. [ Links ]
Rejmánek, M. & D. Richardson. 1996. What attributes make some plant species more invasive?. Ecology 77: 1655-1661. [ Links ]
Sallabanks, R. 1992. Fruit fate, frugivory, and fruit characteristics: a study of the hawthorn, Crataegus monogyna (Rosaseae). Oecologia 91: 296-304. [ Links ]
Sargent, S. 1990. Neighborhood effects on fruit removal by birds: a field experiment with Virbunum dentatum (Caprifoliaceae). Ecology 71: 1289-1298. [ Links ]
Schupp, E.W. 1988. Seed and early seedling predation in the forest understory and in treefall gaps. Oikos 51: 71-78. [ Links ]
Simberloff, D. 1997. Eradication, pp. 221- 228. In D. Simberloff, D.C. Schmitz & T.C. Brown (eds.). Strangers in paradise: Impact and management of non-indigenous species in Florida. Island, Washington, D.C. USA.
Stiles, E.W. 1980. Patterns of fruit presentation and seed dispersal in bird-diseminated woody plants in the eastern deciduous forest. Am. Nat. 116: 670-688. [ Links ]
Uhl, C., E. Buschbacher & E.A.S. Serrao. 1988. Abandoned pastures in eastern Amazonia. I. Patterns of plant succession. J. Ecol. 73: 663-681. [ Links ]
Van der Meer, P.J., F.J. Sterck & F. Bongers. 1998. Tree seedling performance in canopy gaps in a tropical rain forest at Nouragues, French Guiana. J. Tropical Ecol. 14: 119-137. [ Links ]
Vilá, M. & C.M. DAntonio. 1998. Fruit choice and seed dispersal of invasive vs. noninvasive Carpobrotus (Aizoaceae) in coastal California. Ecology 79: 1053-1060. [ Links ]
Vitousek, P.M. & L. Walker. 1989. Biological invasion by Myrica faya in Hawaii: Plant demography, nitrogen fixation, ecosystem effects. Ecol. Monogr. 59: 247-265. [ Links ]
William-Linera, G. 1993. Vegetación de bordes en un bosque nublado en el Parque Ecológico Clavijero, Xalapa, Veracruz, Mexico. Rev. Biol. Trop. 41: 443-453.
1. Dept. of Zoology, PO Box 7617, North Carolina State University, Raleigh, NC 27695-7617 and LIEY. CC 34, 4107 Yerba Buena, Tucumán, Argentina.
2. Present address: IFEVA, Departamento de Ecología, Facultad de Agronomía, Universidad de Buenos Aires, Av. San Martín 4453, 1417 Buenos Aires, Argentina; aragon@ifeva.edu.ar
3. Present address: Dept. of Zoology, P.O. Box 351800, University of Washington, Seattle, WA 98195-1800.
* Corresponding author














