Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.48 n.1 San José Mar. 2000
Abstract
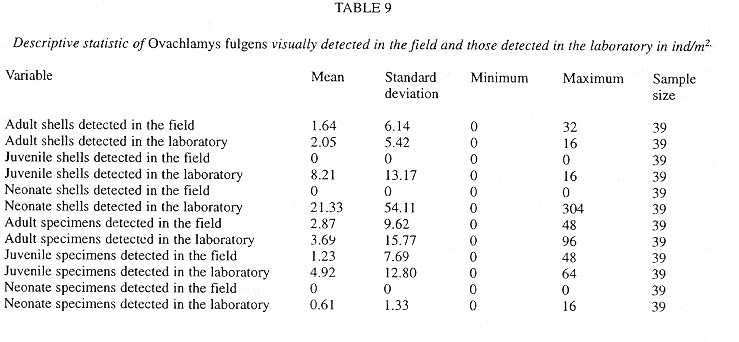
The introduced snail Ovachlamys fulgens (Stylommatophora: Helicarionidae) occurs on cultivated land habitats in Costa Rica, where its macrodistribution seems to be limited by annual mean temperature (20 - 27.6°C) and annual precipitation (1 530 - 3 034 and 3 420 - 8 000 mm, with no more than six dry months). This species can be found in litter and on vegetation up to 70 cm tall. Random quadrat field sampling was done in leaf litter and understory plants every three months for a total of five dates in Central Costa Rica. At least 150 plots of 25x25 cm were analyzed on each date. Abundance of living specimens and eggs was positively correlated with (1) litter abundance and depth, (2) litter and soil humidity, (3) relative humidity and (4) early morning temperature (6:30 AM), and negatively correlated with temperature later in the morning (10:00 AM). Besides these factors, living snail abundance was correlated with thickness of the herbaceous vegetation and with the occurrence of Yucca elephantiphes (in litter and understory). Egg abundance was also correlated with the sampling date, apparently because of changes in humidity. The correlation pattern of shell abundance was opposite to that of living specimens. Population size and number of empty shells throughout the year parallel the rainfall pattern. Reproduction takes place between May and November (wet season); and up to 92% of the specimens can be found aestivating between December and April (dry season). Clutch size averages three eggs. The maximum density of living specimens was reached in December (43.41 ind/m2) and the minimum in March (8.30 ind/m2). Shells decompose in an average of five months.
Key words
Land snail, distribution, microdistribution, Ovachlamys, Helicarionidae, reproduction, demography, shell decomposition, Costa Rica.
Macrodistribution:
The geographic distribution was plotted from the database of the Department of Malacology, Instituto Nacional de Biodiversidad (INBio) in Heredia, Costa Rica. Collecting sites include forested and cultivated areas.
Microdistribution:
Study area: Field work was done in an urban lot (12x18m2) in Pavas, San José, Costa Rica (9o56'45"N, 84o07'15"W, 1075 masl). The biotic area classification is “subtropical, tropical, moist with 5-6 dry months” (Herrera and Gómez 1993 ). The soil is slightly alkaline (pH = 6.6) and calcium-rich (Ca+ =17.1 cmol(+)/l) (Centro de Investigaciones Agronómicas, Universidad de Costa Rica). The site, originally cleared for a coffee plantation, became an urban lot with secondary growth and grasses (Pennisetum purpureum ). In 1982 an orchard was planted, mainly: Persea americana (avocado), Citrus spp. (lemon, tangerine, sour orange), Mangifera indica (mango), Musa sp. (plantain), Psidium guajava (guava), Psidium friedrichsthalianum (sour guava), Yucca elephantipes (yucca), Annona muricata (guanabana) and Acnistus arborescens (güitite). The only maintenance was mechanical mowing (about four times a year). The site was selected because it was the only Costa Rican habitat of O. fulgens known when the study began.
Field methods:
Considering the body size of O. fulgens, site characteristics, ecological variables and time availability, the lot was divided into 3 984 quadrats (25x25cm2). Sample size was determined by preliminary sampling and the equation presented by Southwood (1978) . Five samplings of at least 150 quadrats each were done every three months with the assistance of a random number generator. Sampling was done in the following dates:
1= December 18; 1992 (beginning of dry season), 159 quadrats.
2= March 6-14; 1993 (dry season), 153 quadrats.3= June 8-18; 1993 (wet season), 150 quadrats.4= September 7-14; 1993 (wet season), 150 quadrats.1= December 7-19; 1993 (beginning of dry season), 150 quadrats.
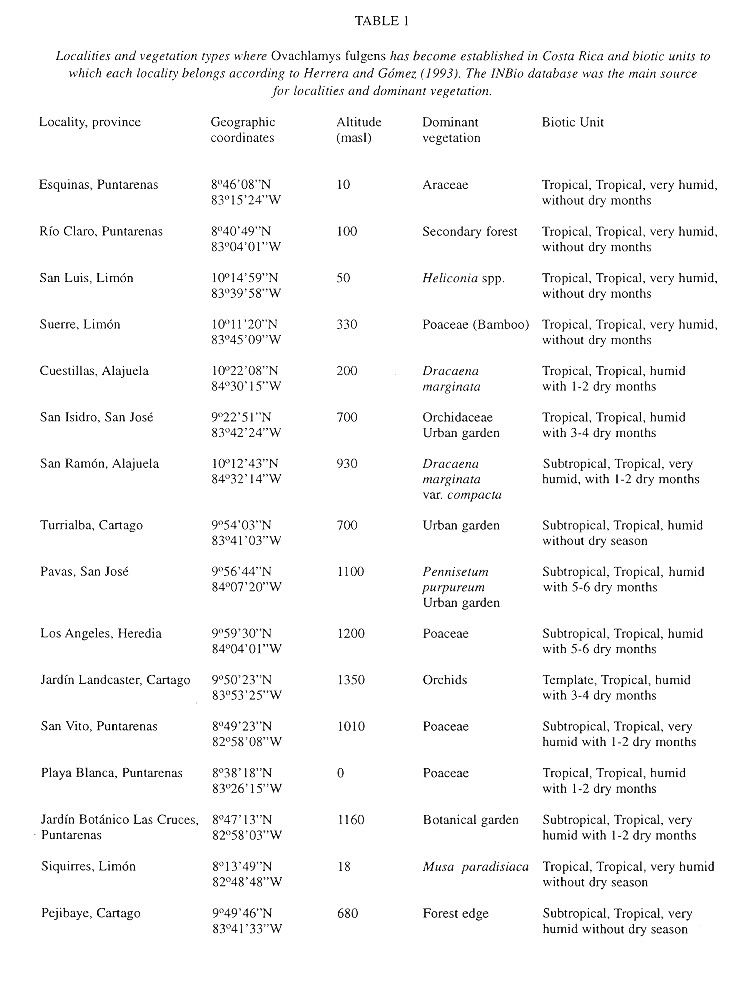
Macrodistribution: The distribution of O. fulgens in Costa Rica (Fig. 1 ) is correlated with human presence and specially with agriculture ( Table 1 ). Only in Pejibaye de Cartago and in Río Claro de Puntarenas ( Table 1 ) was it found in a secondary forest, other samples were collected in gardens or plantations. In Fig. 1 , specimens collected in San Ramón (see Table 1 ) seem to be inside a forested area, but it is crossed over by a road where plantations occur on both sides.
According to Herrera and Gómez (1993) the biotic units where O. fulgens has become established are “tropical”, “subtropical” and “temperate” thermic provinces with the following annual means: 20-27.6oC, minimum 14.8-22.2oC and maximum 24.3-33.1 oC. These are “humid” and “very humid” provinces with a hydric index of 20-300% and with precipitation that ranges between 1 530 - 3 034 mm and 3 420 - 8 000 mm and no more than six dry months (Herrera and Gómez 1993 ).
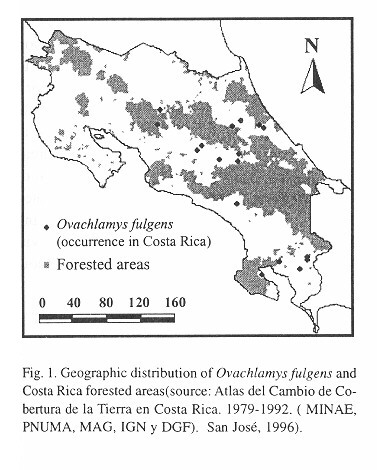
Microdistribution: For equivalent horizontal level and shading conditions, temperatures are higher at 10:00 than at 06:30 hours (Table 2 ). At 06:30 hours locations with trees have higher temperatures than those without trees, but the reverse is true at 10:00 hours ( Table 2 ). The temperature depends on time of day and tree cover ( Table 2 ). At 10:00, everywhere the temperature decreases in the following sequence: air > herbaceous vegetation > litter > soil. At 06:30 hours locations with trees have this temperature sequence: soil > litter > herbaceous vegetation > air. In the tree-less locations the sequence at this time is: soil > litter > air > herbaceous vegetation ( Table 2 ). The thermically most stable location is the soil under trees, followed by soil without trees, litter with trees, herbaceous vegetation with trees, air in locations with trees, litter without trees, herbaceous vegetation without trees and finally the least stable temperatures were recorded in air from the tree-less locations.
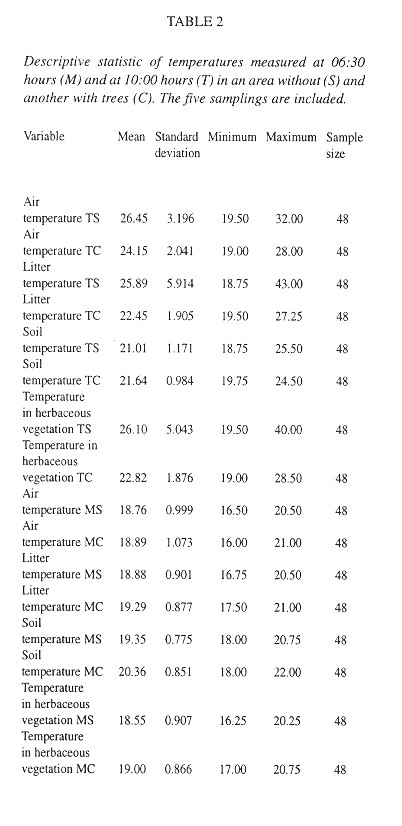
Quadrats were almost (95 %) completely covered by litter, but only 54 % of them had herbs ( Table 3 ). The litter has twice as much humidity as the soil ( Table 3 ). Relatively few quadrats had visible fungal hyphae and these were almost exclusively in the lower litter layers (Table 4 ). The herbs were mainly P. purpureum, Impatiens walleriana and Blechum brownei. (Table 5 ). Litter was dominated by grass blades and leaves of P. americana and P. friedrichsthalianum (Table 6 ).
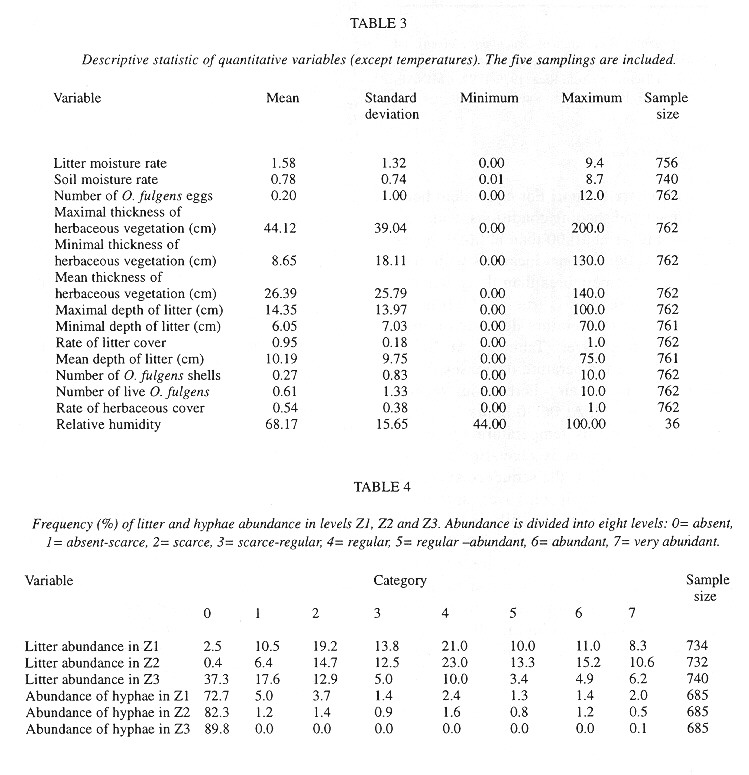
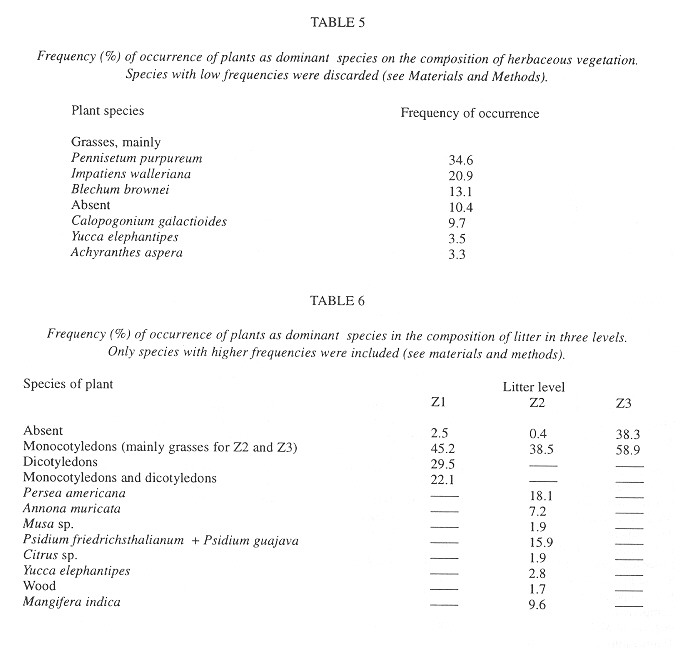
Eggs were found in soil crevices 1 cm deep or less or in the litter closest to the soil. Egg abundance was strongly correlated with litter abundance in Z1 and Z2, litter and soil humidity, litter depth, sampling date and relative humidity. The egg occurrence was: 1- negatively correlated with the temperature in herbaceous vegetation of an area with trees at 10:00 am and 2- positively correlated with the temperature at 6:30 am in the litter of areas with trees and without trees and in the soil in an area without trees ( Table 7 ).
The factors most strongly (negatively) correlated with the presence of shells were the temperature at 6:30 am in the soil and litter of areas with trees and in the soil of areas without trees (Table 7 ).
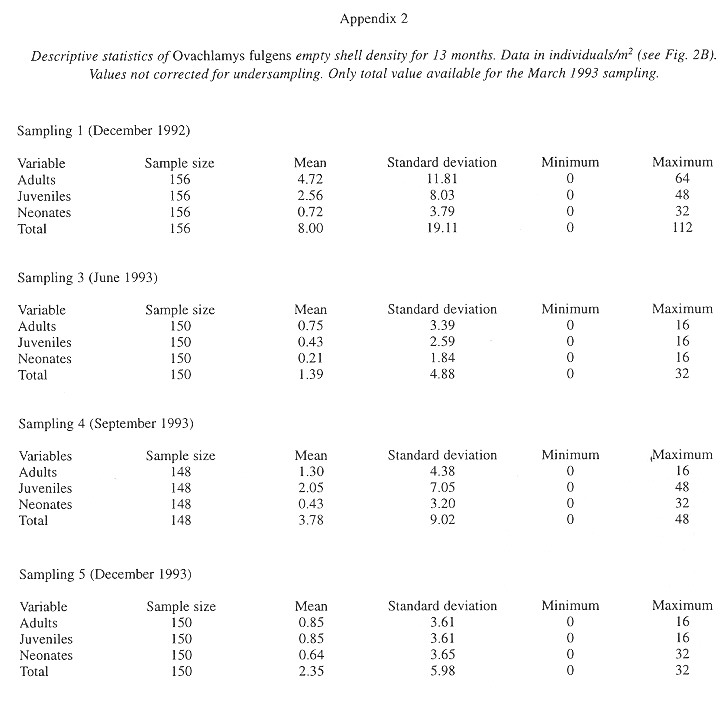
Demography: The small sample size prevented statistic analysis but the general pattern of population dynamics shows correlation with the annual rain cycle (Fig. 2 ), with some time lag. The maximum temperature also seems to have influence on the population dynamics. December 1992 was a very dry and cool month as well as the onset of the dry season, snail population was high, but many aestivating individuals were found. Similarly, the population increase became noteworthy shortly after the first rains. March 1993, although wetter less dry, had the highest temperature of the year and also the fewest amount of live snails. In June 1993 precipitation as well as the snail population rose and the temperature decreased. The same pattern was kept in September 1993.
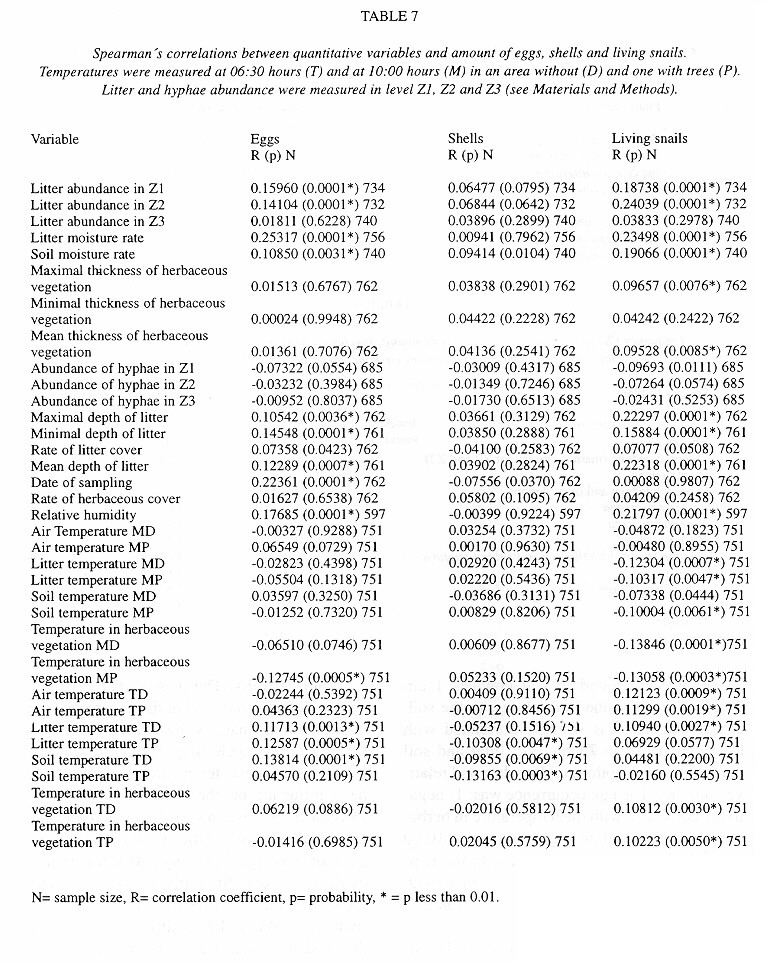
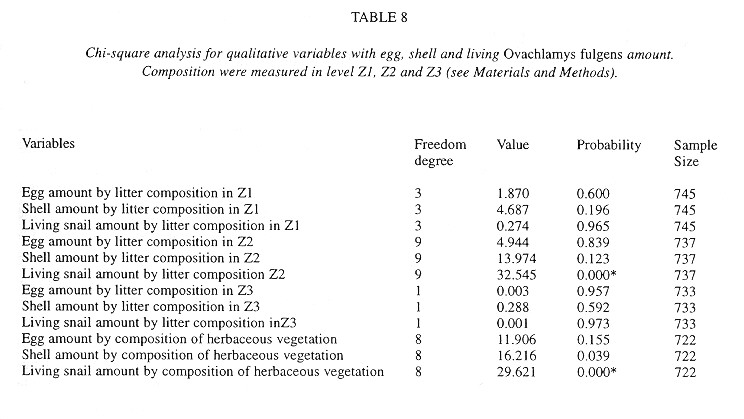
Neonates is the group that during the dry season decreases most and also the one that increases most dramatically (followed by juveniles) with the rains. Neonates and juveniles decrease proportionatelly as they are recruited to the adult class ( Fig. 2 ).
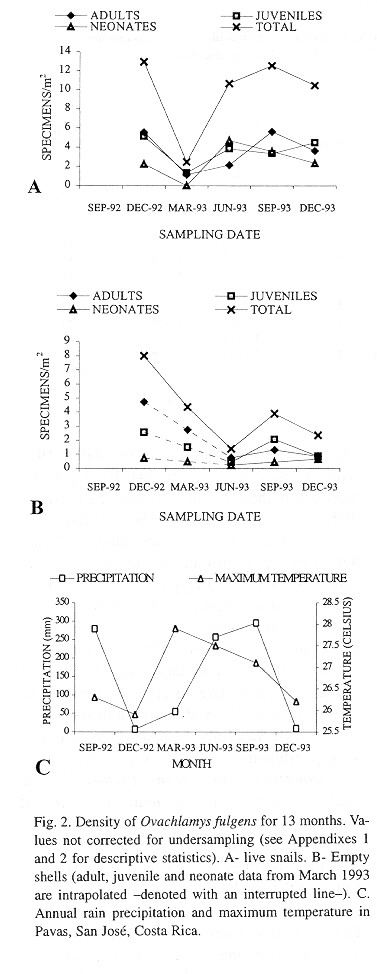
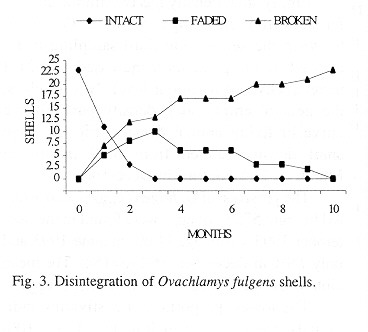
Shell decomposition: Shell decomposition is a gradual process that begins with death. The decomposing soft parts often leave a black stain in the first body whorls; the stain is gradually reduced and finally disappears. At this time the periostracum is not decomposed and the shells are translucent amber in color. This part of the process was labeled "intact" and takes tree months (Fig. 3). Next, the shells "faded", the periostracum is lost, beginning at the apex (only once was a shell totally white before it began to break apart). Then the apex is lost; the process lasts ten months and follows a normal curve (Fig. 3) although the curve ascent occurs in the first three months. The third or "broken" stage occurs after the apex is broken, the shells lose the periostracum and fragments to disappearance. The 23 shells sample required more than ten months for all to disintegrate (Fig. 3); the mean was 147.5 days (23, +102.6, 28-310 days).
Discussion
Macrodistribution: In Costa Rica O. fulgens is correlated with agricultural plantations and with secondary growth vegetation; thus it is probably benefited by deforestation. Nevertheless, it may also become established in forest remnants close to plantations.
The macrodistribution of O. fulgens appears to be limited by temperature and humidity because it has not been found in regions with annual means below 20oC and above 27.6oC and annual precipitation below 1530mm. The data from Naha (the only city in the Liu Chiu Islands with meteorological data available) fully agree with these ranges. For this reason, the species will probably fail to colonize the northern Pacific region (Nicoya, basically), and the highlands above 1700masl. The only biotic zones still open to establishment are those classified as "temperate, tropical very humid" mainly on the bases of the mountain ranges in the center and south of Costa Rica.
Microdistribution: According to this study the number of eggs and live snails of O. fulgens increase with the increase of temperature during the morning (6:30 am) and with the decrease of temperature later in the morning (10:00am) showing a tendency to thermic stability. Exactly the opposite situation was found in the case of empty shell, a fact which supports the last conclusion. Apparently the thermic stability that tree cover gives is not significant for the abundance of O. fulgens, making the species more suitable to colonize cultivated areas. Similarly to the findings of Boag (1985) , thermic stability appears to determine substrate selection of adults.
Other factors clearly correlated with occurrence of eggs and live snails were humidity of litter and soil and season (for eggs only), as reported for temperate species (Boag 1985 , Locasciulli and Boag 1987 , Nilsson et al. 1988 ). High relative humidity is also an important factor for snail and egg abundance, an expected result due to the hygroscopic nature of the eggs and to the snails’ slime trail production. This study suggests that O. fulgens lays eggs in the soil probably not only because it is, thermically, the most stable microhabitat available, but also because these can take advantage of the increased moisture during the rainy season (see Barrientos 1998 ). Degen et al. 1992 found that the desert snail Trochoidea seetzenii markedly selects microhabitats with stable humidity, but this study suggests that O. fulgens prefers moister soil and litter, not the more stable. The results of this study do not support Peake`s (1968) opinion that well drained soils favor the presence of molluscs.
Litter depth and abundance also affected eggs and living O. fulgens abundance. Abramsky et al. (1990 , 1992 ) stated that the factors determining abundance of desert species are predation, oviposition sites and habitat heterogeneity. Brown and Lodge (1993) stressed the relationship between abundance and habitat structural complexity, the latter increases the area that can be colonized. In the present study, the factors listed by Abramsky et al. (1990 , 1992 ) and Brown and Lodge (1993) are the result of litter depth and abundance because thicker litter has more sites to colonize, lay eggs in and hide from predators.
Resumen
El caracol introducido Ovachlamys fulgens (Stylommatophora: Helicarionidae) habita regiones cultivadas de Costa Rica y su distribución está limitada por temperaturas anuales entre 20 y 27.6oC y precipitaciones anuales entre 1530-3034 y 3420-8000 mm con no más de seis meses secos. Esta especie habita en la hojarasca y en la cobertura herbácea hasta a 70 cm de altura. Se realizaron cinco muestreos; uno cada tres meses. En cada muestreo se analizaron un mínimo de 150 parcelas aleatorias de 25 x25 cm. Los factores que determinan la abundancia de individuos y huevos fueron la abundancia y profundidad de la hojarasca, la humedad del mantillo y del suelo, y la humedad relativa. Éstos a su vez estuvieron positivamente correlacionados con la temperatura a primeras horas de la mañana (6:30 am) y negativamente con la temperatura a media mañana (10:00 am). Aparte de estos factores, los caracoles tambien estuvieron correlacionados con el grosor de la capa herbácea y con la presencia deYucca elephantipes tanto en el mantillo como en la vegetación. Los huevos tambien se vieron afectados por la época del año, debido a su propiedad higroscópica. La abundancia de conchas se correlacionó únicamente con la temperatura, en patrón contrario al de los los huevos. Tanto la población como la cantidad de conchas siguieron, con un leve retraso, la curva de lluvias de la zona a lo largo del año. La época reproductiva abarca los meses de mayo a noviembre (época lluviosa); y durante los meses de diciembre a abril (época seca) se puede encontrar hasta el 92% de los especímenes estivando. En condiciones naturales la camada promedio tiene tres huevos. Los caracoles vivos tuvieron una densidad máxima de 12.92 ind/m2 (en diciembre) y mínima de 2.47 ind/m2 (en marzo; estos valores deben multiplicarse por el factor de corrección 3.36). En condiciones experimentales las conchas tardaron intactas dos meses y su descomposición total se produjo en cinco meses en promedio.
Acknowledgements
This study was financed by the author, by Consejo Nacional para Investigaciones Científicas y Tecnológicas (CONICIT) project 94-520-BID and by Netherlands Development Assistance (NEDA). Instituto Nacional de Biodiversidad (INBio) provided time and facilities to finish it. I thank Fernando Barrientos H. and Lilia Llosa for allowing work on their property. Fernando Barrientos Ll., José Franco (Coalición Nacional de Iniciativas de Desarrollo), Vernon Arias (Universidad de Costa Rica: UCR), Richard Helling, Carlos Porras, Julio Magaña (INBio), Marianella Segura (INBio), Franscisco Quesada (INBio), Mario Chinchilla (INBio), Eida Fletes (INBio), Kattya Quesada (INBio), Socorro Avila (INBio), Marcos Castro (INBio) and Luis Murcia (INBio) assisted field and laboratory work. Plant identification was done by Quírico Jiménez (INBio), Carlos Morales (UCR) and Nelson Zamora (INBio). The departments of genetics and entomology of Escuela de Biología (UCR) lent some equipment. David Boag, Marco de la Torre and two other anonymous reviewers read the manuscript and improved the language. María Isabel González (UCR) and Juan Bautista Chavarría (UCR) provided statistical assistance and Randall Wong made the statistical analysis. I specially thank Fred G. Thompson (University of Florida) for taxonomic advice and Julián Monge-Nájera (UCR) for providing unconditional assistance in several stages of this paper. This manuscript is part of a M.Sc. thesis presented at the University of Costa Rica.
References
Abramsky, Z., H. Alfia, M. Shachak & S. Brand. 1990. Predation by rodents and the distribution and abundance of the snail Trochoidea seetzenii in the Central Negev Desert of Israel. Oikos 59: 225-235. [ Links ]
Abramsky, Z., M. Shachak, A. Subach, S. Brand & H. Alfia. 1992. Predator-prey relationships: Rodent-snail interactions in the Central Negev Desert of Israel. Oikos 65: 128-133. [ Links ]
Alonso S., M.A. & V. Berovides A. 1991. Densidad y variación morfológica de Zachrysia guanensis (Gastropoda: Camaenidae) en Pinar del Río, Cuba. Rev. Biológica 5: 97- 105. [ Links ]
Barrientos, Z. 1996. Ciclo de vida y comportamiento del caracol terrestre Ovachlamys fulgens (Gude, 1900) (Stylommatophora: Helicarionidae) en el laboratorio. Tesis de Maestría, Universidad de Costa Rica, San José (cap. 2). [ Links ]
Barrientos, Z. 1998. Life history of the terrestrial snail Ovachlamys fulgens (Stylommatophora: Helicarionidae) under laboratroy conditions. Rev. Biol. Trop. 46(2): 369-384. [ Links ]
Bidart, L., M. Osorio & E. Reinaldo. 1992. Ecología de Polymita muscarum en la localidad ¨El Yayal¨, provincia de Holguín. Reporte de investigación del Instituto de Ecología and Sistemática. Academia de Ciencia de Cuba. Ser. Zool. 17: 3-14. [ Links ]
Bishton, G. 1986. The diet and foraging behavior of the Dunnock Prunella modularis in a hedgerow habitat. Ibis 128: 526-539. [ Links ]
Boag, D.A. 1985. Microdistribution of three genera of small terrestrial snails (Stylommatophora: Pulmonata). Can. J. Zool. 63: 1089-1095. [ Links ]
Boag, D.A. & W.D. Wishart. 1982. Distribution and abundance of terrestrial gastropods on a winter range of bighorn sheep in southwestern Alberta. Can. J. Zool. 60: 2633-2640. [ Links ]
Brown, K. & D.M. Lodge. 1993. Gastropod abundance in vegetated habitats: The importance of specifying null models. Limnol. Oceanogr. 38: 217-225. [ Links ]
Chatfield, J. E. 1975. A summary on studies on food and feeding in some European land snails. Malacol. Review 8: 123-125. [ Links ]
Coppois, G. 1984. Distribution of bulimulid land snails on the northern slope of Santa Cruz Island, Galapagos. Biol. J. Linn. Soc. 21: 217-227. [ Links ]
Dallas, H.F., B.A. Curtis & D. Ward. 1991. Water exchange, temperature tolerance, oxygen consumption and activity of the Namib Desert snail, Trigonephrus sp. J. Moluscan Stud. 57: 359-366. [ Links ]
Degen, A.A., A. Leeper & M. Shachak. 1992. The effect of slope direction and population density on water influx in a desert snail, Trochoidea seetzenii. Funct. Ecol. 6: 160-166. [ Links ]
Digweed, S.C. 1993. Selection of terrestrial gastropod prey by cychrine and pterostichine ground beetles (Coleoptera: Carabidae). Can. Entomol. 125: 463-472. [ Links ]
Emberton, K.C., T.A. Pearce & R. Randalana. 1996. Quantitatively sampling land-snail species richness in Madagascan rainforests. Malacologia 38: 203-212. [ Links ]
Gotwald, W.H.Jr. 1972. Analogous prey escape mechanisms in a pulmonate mollusk and lepidopterous larvae. N. AND. Entomol. Soc. 80: 111-113. [ Links ]
Gude, G.K. 1900. Further notes on Helicoid Land Shells from Japan, The Loo-Choo, and Bonin Islands, with descriptions of seven new species. Proc. Malacol. Soc. 4: 70-80. [ Links ]
Herrera S., W. & L. D. Gómez P. 1993. Mapa de Unidades Bióticas de Costa Rica. Incafo S.A. et al. San José, Costa Rica. 1: 685 000. [ Links ]
Hodgson, A.N. & M. Shachak. 1991. The spermatogenic cycle and role of the hermaphrodite duct in sperm storage in two desert snails. Invertebr. Reprod. Dev. 20: 125-136. [ Links ]
Hyman, L.H. 1967. The invertebrates Vol. 6: Mollusca I. McGraw-Hill, New York. 791p. [ Links ]
Kralka, R. 1986. Population characteristics of terrestrial gastropods in boreal forest habitats. Am. Nidl. Nat. 115: 156-164. [ Links ]
Laurens, A., J. Belot & C. Delorme. 1987. Molluscicidal activity of Anacardium occidentale L. (Anacardiaceae). Ann. Pharm. Fr. 45: 471-473. [ Links ]
Lazaridou-Dimitriadou, M. & D.S. Sauders. 1986. The influence of humidity, photoperiod, and temperature on the dormancy and activity of Helix lucorum L. (Gastropoda, Pulmonata) J. Molluscan Stud. 52: 180-189. [ Links ]
Locasciulli, O. & D.A. Boag. 1987. Microdistribution of terrestrial snails (Stylommatophora) in forest litter. Can. Field-Nat. 101: 76-81. [ Links ]
Molgaard, P. 1986. Food plant preferences by slugs and snails: A simple method to evaluate the relative palatability of the food plants. Biochem. Syst. Ecol. 14: 113-122. [ Links ]
Monge-Nájera, J. 1996. Molluscs of economic and sanitary importance in the Tropics The Costa Rican experience. Universidad de Costa Rica, San José. 166p. [ Links ]
Nilsson, S.G., J. Bengtsson & S. As. 1988. Habitat diversity or area per se? Species richness of woody plants, carabid beetles and land snails on islands. J. Anim. Ecol. 57: 685-704. [ Links ]
Orians, G.H., R. Dirzo & J.H. Cushman (eds.). 1996. Biodiversity and Ecosystem Processes in Tropical Forests. Springer, Berlin. (Ecological Studies Vol. 122) 229 p. [ Links ]
Peake, J. F. 1968. Habitat distribution of Solomon Island Land Mollusca. Symp. zool. Soc. London 22: 319-346. [ Links ]
Reichholf, J. 1986. Density, activity and composition of a local population of the bush snail Bradybaena similaris in southern Brazil. Mitt. Dtsch. Malakozool. Ges. 0(38): 31-44. [ Links ]
Sirby, D.F. 1984. Food of the sand lizard (Lacerta agilis). Stud. Univ. Babes-Bolyai Biol. 29: 18-21. [ Links ]
Solem, A., S. Tillier & P. Mordan. 1984. Pseudo-operculate pulmonate land snails from New Caledonia. The Veliger 27: 193-199. [ Links ]
Southwood, T.R.E. 1978. Ecological Methods. Chapman and Hall, Cambridge. 524p. [ Links ]
Speiser, B. & M. Rowell-Rahier. 1991. Effects of food availability, nutritional value, and alkaloids on food choice in the generalist herbivore Ariantaarbustorum (Gastropoda: Helicidae). Oikos 62: 306-318. [ Links ]
Stamol, V. 1993. The influence of the ecological characteristics of phytocoenoses on the percentage proportions of zoogeographical elements in the malacocoenoses of land snails (Mollusca: Gastropoda terrestria). Vegetatio 109: 71-80. [ Links ]
Van der Laan, K.L. 1981. Terrestrial pulmonate reproduction: seasonal and annual variation and environmental factors in Helminthoglypata arrosa (Binney) (Pulmonata: Helicidae). The Veliger 23: 48-54. [ Links ]
Van Es, J. & D.A. Boag. 1981. Terrestrial molluscs of central Alberta. Can. Field-Nat. 95: 75-79. [ Links ]
Villalobos M., C., J. Monge-Nájera, Z. Barrientos & J. Franco. 1995. Life cycle and field abundance of the snail Succinea costaricana (Stylommatophora: Succineidae), a tropical pest. Rev. Biol. Trop. 43: 181-188. [ Links ]
Waite, T.A. 1987. Behavioral control of water loss in the terrestrial slug Deroceras reticulatum (Müller): Body-size constraints. The Veliger 30: 134-137. [ Links ]
Ward, D. & R. Slotow. 1992. The effects of water availability on the life history of the desert snail, Trochoidea seetzeni. Oecologia 90: 572-580. [ Links ]
1 Departamento de Malacología, Instituto Nacional de Biodiversidad (INBio), Apdo. 22-3100 Sto. Domingo, Heredia, Costa Rica. Fax (506)2442816, E-mail: zbarr@inbio.ac.cr














