Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.46 n.3 San José Sep. 1998
Copulation, fighting behavior and life cycle of Neopamera bilobata
(Heteroptera: Lygaeidae)
Rafael Lucas Rodríguez S 1
Received 6-XI-1997. Corrected 13-IV-1998. Accepted 15-IV-1998.
Resumen
Durante una cópula de Neopamera bilobata, el macho cortejó a la hembra con sacudidas verticales de su cuerpo, y ella lo empujó con sus patas posteriores. Dos machos pelearon una vez con sus patas delanteras, las cuales tienen el fémur engrosado y con espinas. Una hembra depositó 2.2 huevos/día, y las ninfas tardaron 10-12 días en eclosionar. Un macho y una hembra criados en el laboratorio vivieron por 52 y 45 días, respectivamente.
Key words
Lygaeidae, copulation behavior, male fights, Neopamera bilobata, reproduction.
Many members of the Lygaeidae (Heteroptera) are seed predators of figs (Moraceae: Ficus) (Slater 1972). Little is known about their natural history in the tropics. This paper presents data on the natural history and behavior in Costa Rica of Neopamera bilobata Say (Heteroptera: Lygaeidae), a general seed feeder found on a wide variety of plants in disturbed habitats (J. A. Slater, pers. comm.).
Six nymphs were found near San Antonio de Escazú (el. 1300-1400 m), San José Province, Costa Rica, in an area of coffee plantations and secondary forest. They were on the ground below two adjacent Ficus padifolia H.B.K. (= F. pertussa, sensu Burger) trees, where fig fruits had recently fallen. The nymphs were kept in Petri dishes, and fed ripe fig fruits with their seeds, and sunflower seeds. Fruits were kept in the freezer before use, then thawed and dried, and discarded from the Petri dishes every 2-4 days. Water was provided in a glass vial stoppered with cotton, and fixed in the Petri dish with plasticine. A piece of cork impregnated with fungicide (Nipagin) was kept in each Petri dish to form a complex, three dimensional environment to favor nymphal development (Coulianos and Kugelberg 1973). Mating and fighting behavior were observed under the dissecting microscope. Adult bugs were placed in a Petri dish on top of a mirror, and their behavior was recorded with taped verbal descriptions. The female ovipositor is semi-transparent, so some genitalic events were observed during mating. A mated female was kept in a Petri dish with food and water until her death, and the number, length, fertility (change in color from white to red), and eclosion of the eggs laid were noted daily. Averages are given (the standard error. Voucher specimens were deposited at INBio (Instituto Nacional de Biodiversidad, Santo Domingo de Heredia, Costa Rica).
One copulation of virgin individuals was observed. When they met, the male and female began to vibrate their antennae vertically. The female stopped at the beginning of copulation, resumed after 3 min, and continued until the end. The male vibrated his antennae throughout copulation. He mounted the female several times facing in her direction while vibrating his antennae, and tapped her ovipositor with his genital capsule. During these mounts, the female moved her hind legs backwards, rubbing her wings and abdomen, and pushing his abdomen and genital capsule with her tibiae and tarsi. Copulation began after about 3 min and several mounts, and lasted 45.4 min. After genital coupling, the male dismounted, and turned to face in the opposite direction, so that his genital capsule was twisted 180°. His endophallus and sperm reservoir then went through the female's ovipositor, and were lost from sight within her abdomen. Later, the male's genitalia emerged from the female's abdomen, moved back and forth between the ovipositor and genital capsule, and then re-entered through the ovipositor. This happenned twice during the 4th min of copulation, each time for 10 s. The female's ovipositor moved slightly back and forth at the tip of the male's genital capsule for 20 s during the 4th min of copulation, and for 3 min after 30 min of copulation. During the last 2 min, there was a flow of material to the female going through her ovipositor. The female pushed the male with her hind legs in 12 episodes of 22 + 7 s each. In all, she pushed him for 262 s. He continuously made 1-2 strong vertical jerking movements per second with his whole body, jerking her. Copulation ended on a moment in which the female was pushing the male. After 10 min, she ejected a spermatophore full of living sperm.
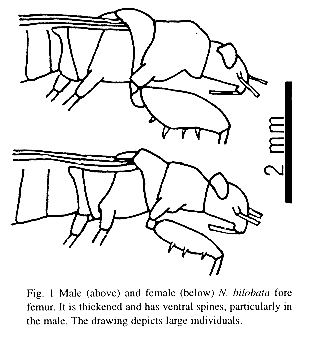
An aggressive encounter between two males was observed. When they met they began to vibrate their antennae, and then fought for 20 s. They placed themselves head to head, rose on their hind legs, and struck each other repeatedly with their fore legs, which have thickened femora and ventral spines (Fig. 1). Each striking movement began with both fore legs extended laterally and parallel to the substrate. The legs swung anteriorly and struck the opponent with the femur and tibia. The fight ended when one male walked away, and one male walked away from the other on subsequent encounters. There were no aggressive interactions between females.
The mated female lived for 76-79 days, 69 after mating, and laid on average 2.2 eggs/day until her death. Only eggs laid during the first 40 days were fertile. Of the 155 eggs laid, 64 % were fertile, and nymphs eclosed from 63 % of those. Egg length was 0.98 + 0.01 mm (n = 128), and did not differ significantly between fertile and infertile eggs (Mann Whitney U, p = 0.07). Fertile eggs began to change color after 4.4 + 0.1 days (n = 17 maturation periods of eggs laid on a given day), and were fully red after 6.4 + 0.3 days (n = 15 maturation periods). Nymphs eclosed after 10-12 days. The female laid 95% of her eggs on the open fig fruits, and 97% on days in which she had food. As food was accessible to her only on alternating days, the above Figs. are probably underestimates of her capacities. When she died she had 15 mature and no immature eggs inside. One lab reared male and a female lived as adults for 52 and 45 days, respectively.
Male jerking movements during copulation fit most criteria to be considered copulatory courtship (Eberhard 1994). They were stereotyped, appropiate to stimulate the female, apparently irrelevant for genital coupling, and occurred repeatedly during copulation. Not so with antennal vibration, which also occurred in intra-sexual encounters. The more likely function of male copulatory courtship is to affect cryptic female choice (Eberhard 1994, 1996). The ejection of a spermatophore full of living sperm by a once-mated female suggests N. bilobata females may exert such choice.
Females touch the male during copulation in several species of true bugs (Table 1). In the lygaeid Ozophora baranowskii, tapping appears to selectively reject some males during copulation (Rodríguez 1996), but it may be a forceful way to end copulation in Oncopeltus fasciatus (Loher and Gordon 1968). The data presented here can not discriminate between these hypothesis for N. bilobata.
The N. bilobata fight described here is similar to Sweets (1964) description for Pachybrachius bilobatus (= N. bilobata). Fighting behavior and sexual dimorphism suggest the fore femur of N. bilobata may be under sexual selection, but this is not a likely explanation for the thick, spiny fore femur commonly found in true bugs, especially rhyparochrominae lygaeids (Schuh and Slater 1995). Male fights appear to be rare in these bugs. Sweet (1964) saw them in only two other species, Pachybrachius basalis and Sisamnes contractus (= Exptochiomera antennata), and suggested fights might be observed more frequently under "proper" conditions. But there were no fights in over 120 interactions of males and females of two Ozophora species observed with and without seeds (Rodríguez 1996), although there were many male-male encounters, and their fore femur is spiny (but not sexually dimorphic). A general explanation for male and female thick, spiny fore legs would be to aid the rostrum in moving large seeds on substrates with obstacles (Sweet 1964).
I thank William G. Eberhard for access to his laboratory, and comments on the manuscript. Daniel Briceño, Paul Hanson, Bernhard Huber, and two anonymous reviewers commented on the manuscript. James A. Slater kindly identified the bugs, provided literature, and commented on the manuscript. William Ramírez kindly identified the fig. My family provided financial support.
TABLE 1
Known instances of females that touch and that may stimulate the male during copulation in the Heteroptera
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
References
Coulianos C.-C. & O. Kugelberg. 1973. A simple method for rearing terrestrial Heteroptera, with special reference to seed-bugs (Het. Lygaeidae). Ent. Scand. 4: 105-110. [ Links ]
Eberhard W. G. 1994. Evidence for widespread courtship during copulation in 131 species of insects and spiders, and implications for cryptic female choice. Evolution 48: 711-733. [ Links ]
Eberhard W. G. 1996. Female control: sexual selection and cryptic female choice.Princeton, New Jersey. 501 p. [ Links ]
Loher W. & T. H. Gordon. 1968. The maturation of sexual behaviour in a new strain of the large milkweed bug, Oncopeltus fasciatus. Ann. ent Soc. Am. 61: 1566-1572. [ Links ]
Rodríguez S., R. L. 1996. Biología sexual de dos abejones (Coleoptera: Cicindelidae) y tres chinches (Heteroptera: Lygaeidae). M. Sc. Thesis, University of Costa Rica, San José, Costa Rica. [ Links ]
Schuh, R.T. & J. A. Slater. 1995. True bugs of the World. Comstock, Ithaca, New York. 336 p. [ Links ]
Slater, J. A. 1972. Lygaeid bugs (Hemiptera: Lygaeidae) as seed predators of figs. Biotropica 4: 145-151. [ Links ]
Sweet MH. 1964. The biology and ecology of the Rhyparochrominae of New England. Entomol. Amer. 43: 1-124. [ Links ]
1Escuela de Biología, Universidad de Costa Rica, 2060, San José, Costa Rica