Introduction
The aim of the endodontic treatment is to eliminate the root canal's infection and necrotic tissue and fill the three-dimensional space of the root canal system to prevent the passage of liquids and microorganisms (1,2). Root canals are obtura- ted with core materials (commonly gutta-percha) in combination with an endodontic sealer. Hence, a proper root canal sealer must establish a fluid-tight cover between the root canal's dentinal wall, the gutta-percha, and the apex. For many decades, it has been supported that an adequate sealer must not interfere with the healing processes and repair of the apical and periapical tissues (1,2,3).
Although, the obturation materials are intended to remain within the root canal, some of these materials may be pressed beyond the periapical foramina during the obturation process, more commonly the sealer. This may happen due to different situations such as anatomical variations, material's flowability and the selected obturation technique such as thermoplasticized gutta-percha use (4). When a material is extruded from the anatomical apex of the root canal, the cytotoxicity and the biological behavior of the material could influence the treatment outcome. Hence, the selection of the adequate obturation material becomes relevant depending of the initial diagnosis for each individual case. As an example, the regeneration of periapical bone loss caused by chronic inflammation in response to pulpal necrosis may be favored by selecting the materials and clinical techniques that could induce a positive biological response (5).
It has been stated that all root canal sealers, independent of their composition, exhibit some degree of toxicity when they are freshly mixed and this feature may decrease after they are fully set (6). AH-Plus, a resin epoxy-based sealer, is considered the gold standard of endodontic sealers, due to its excellent physicochemical properties (7), though, studies show that materials containing epoxy resin may prompt a cytotoxic response (8,9) and stimulate an inflammatory reaction of the surrounding tissues (10). Root canal sealers have evolved in the recent years, particularly with tricalcium silicate-based sealers also called bioce- ramic sealers (11). These sealers can be presented as a premixed formulation, or as a powder and liquid preparation. The powder is mostly composed of tricalcium silicate and zirconium oxide (opacifier), and the aqueous solution is made of calcium chloride and excipients (12,13). The modified composition of these type of endodontic sealers provides benefits when compared to other traditional sealers, improving their physicochemical and biological properties. Moreover, the periapical structures and the periodontal tissues have displa- yed favorable biological responses and tolerance to tricalcium silicate-based sealers. Also, tricalcium silicate-based sealers release hydroxyapatite during the setting reaction, favoring osteoconductivity and offers antibacterial properties explained by their alkaline pH during the first 24h of setting (12,14,15).
Whereas the flowability property of these tricalcium silicate-based sealers favors the material confinement within the root canal space, extrusion of the sealer may occurs during obturation procedures. The contact between the sealer and the soft and hard periapical tissues can cause continuous inflammation of peri radicular tissues and may result in delayed wound healing. In this regard, periodontal ligament cells represent target cells in endodontic treatments due to their biolo- gical response during apical sealing. Furthermore, the final behavior and placement of each sealer will be related with the obturation technique employed, and not only with the properties offered by each material. Thus, this study aimed to compare the adhesion and proliferation of human periodontal ligament fibroblasts in transverse sections of premolar teeth sealed with a tricalcium silicate-based sealer used with hydraulic obturation (HO) and an epoxy-based sealer used with a continuous-wave condensation (CWC). The experimental root model aimed to simulate the final behavior of each sealer once used with an specific suggested obturation technique; and its response with the periodontal ligament cells.
Materials and methods
Cell culture materials and reagents were obtained from Corning Materials Science Technology and Innovations (New York, USA) and Sigma- Aldrich (Missouri, USA). Root canal sealers BioRoot RCS (Septodont, Saint Maur DesFosses, France) and AH-Plus (Dentsply Maillefer, Switzerland) were selected as the tricalcium silicate-based and the epoxy-based sealers, respectively. All tissue samples were obtained from healthy patients following the guidelines established by the Research and Ethics Committee of the School of Dentistry at the UNAM (Number, CIE/1110/2017), and the Mexican legislation for research in human subjects (NOM-012- SSA3-2012). The written informed consent was obtained from all the patients or their parents.
Cell culture
Human periodontal ligament fibroblast cells (hPDLs) were prepared from immature third molars freshly extracted for orthodontics reasons in compliance with the maxillofacial surgery clinic by the explant outgrowth method (16). Cells were cultured in minimum essential alpha medium (α-MEM) supplemented with 10% fetal bovine serum, 100 UI/mL penicillin, 100 mm/mL strep- tomycin, and 0.25 mg/mL amphotericin B; at 37°C in a 95% air 5% CO2 atmosphere. Also, the supplemented α-MEM medium was used for further experimentation.
Sample preparation
Intact freshly extracted single-rooted maxillary and mandibular premolars were stored at 4°C in saline solution (supplemented with 100 U/mL penicillin, 100 mg/mL streptomycin, and 0.25 mg/mL amphotericin B). A 10 K-file (Dentsply Maillefer, Switzerland) was used to ensure apical patency by visualizing the 10 K-file tips at the root apex and to measure the working length. When apical patency was not attained, a tooth had an open apex or presented more than one canal the tooth was excluded. A total of 30 teeth were selected and K-files of increasing diameter were used to measure the apical diameter at the working length before instrumentation. Root canals were prepared using Protaper Next (Dentsply Maillefer, Switzerland) rotatory system with an endodontic motor with adaptive motion (SybronEndo Elements, USA). The instrumental sequence was as follows: File X1 (size 17, .04 taper), X2 (size 25, .06 taper) and X3 (size 30, .07 taper); using constant irrigation with a 2.5% of sodium hypochlorite solution. Teeth were randomly assigned in 2 experimental groups (n=15) using CWC with AH-Plus + or single cone HO with BioRoot RCS. After obturation, the crowns were removed at the cement-enamel junction using a diamond disk under cold water (Figure 1). Cross-sections of the roots (2mm) were obtained and only 4 sections from the central area of each root were selected. A total of 60 cross-sections were used per group. The specimens were sterilized under ultraviolet light for 15min, and stored for 24h in a 100% humidity incubator at 37°C to allow the setting of the root filling materials. BioRoot RCS and AH-Plus were freshly prepared for each tooth according to manufacturers' instructions.
Cellular adhesion assays
The hPDL cells were seeded at 1×104 cells/mL onto the root slices and placed in 48-well culture plates with complete α-MEM medium, and allowed to adhere under standard cell culture conditions for 4 and 24h. After the prescribed time, the root slices were rinsed three times employing PBS to remove non-adherent cells. The evaluation of cell attachment was performed according to a crystal violet assay (17). Adherent cells were fixed with 4% paraformaldehyde and incubated with 0.1% crystal violet solution for 15min (Figure 1.D). The dye was then extracted with 0.1% of sodium dodecyl sulfate (SDS), and optical absorption was quantified by spectrophotometry at 545nm with a ChroMate, Awareness Technology (Florida, USA) plate reader.
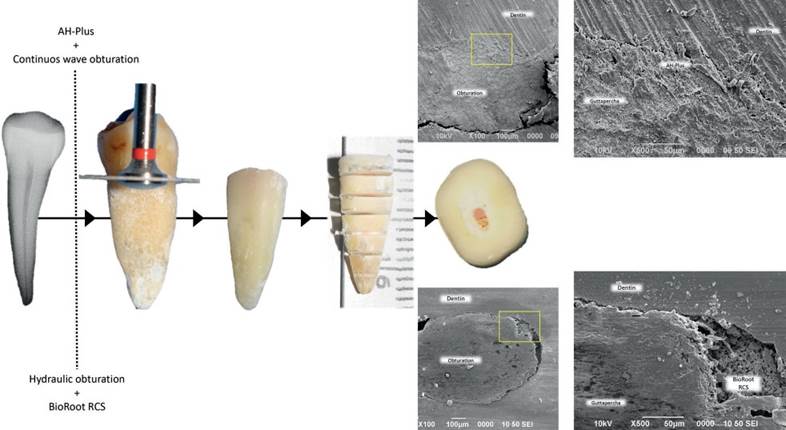
Figure 1 The root model experimental procedure. Crowns were removed at the cement-enamel junction. 30 teeth were selected, instrumented and obturated with Bioroot RCS/Hydraulic obturation or AH-Plus/Continuous-wave condensation. Cross-sections of the roots were made of 2mm to obtain the samples. Representative scanning electron microscopy images of the slices are shown.
Cell proliferation assay
The WST-1 assay was performed to evaluate the cell viability of hPDLs seeded onto the roots at a concentration of 1×104 cells/mL at 3,7,9, 14, and 21 days of culture (18). Briefly, for each time point, the hPDLs seeded onto the root slices were washed with PBS and incubated with 400μL fresh culture medium containing 40μL of the cell proliferation reagent WST-1 for 4h at 37°C. Then, 200μL of the supernatant was removed, and the absorbance was quantified by spectrophotometry at 450nm with a plate reader (Chromate, Awareness Technology, UK). During the time of the experiment, the medium was changed with fresh supplemented medium every 3 days. The experiment was carried out in triplicate, with three repetitions for each condition every time.
Cell morphology evaluation
Specimens were incubated with complete α-MEM for 24h and then evaluated with fluores- cence microscopy. Briefly, before seeding onto the root slices, the hPDL cells were incubated with Cell-Tracker Green CMFDA (5-chloromethyl- fluoresceindiacetate) in phenol red-free medium at 37°C for 30min (19). Subsequently, the cell culture was washed with PBS and incubated for 1h in complete medium. After recovery, hPDLs were trypsinized and counted to the desired cell concentration (1×104 cells/mL) and incubated for 24h onto the root slices. The cells' morphology was subsequently evaluated, particularly their spreading pattern and cell-material interaction.
Results
Adhesion assay
According to the established time intervals, the cell adhesion response of hPDL cells seeded onto tooth slices was analyzed by crystal violet assay (Figure 2). The results showed that more cells adhered to the root slices obturated with BioRoot RCS/HO than on the slices with AH-Plus/CWC. Both groups of treated root slices showed higher adhesion when compared to control after 4 and 24h of cell culture, with an increase response to the BioRoot RCS/HO group (p<0.05). The result suggests that the obturation technique contributes significantly to the attachment of hPDL cells, and the fluorescence staining supported the results regarding cell adhesion.

Figure 2 Results of the crystal violet assay. Cell adhesion of hPDL after 4 and 24h of culture in the presence of BioRoot RCS/hydraulic obturation (HO) and AH-Plus/continuous-wave condensation (CWC). The difference in cell adhesion was statistically significant between control & AH-Plus/CWC (*) between control & BioRoot RCS / HO (**), and between AH-Plus/CWC and & BioRoot RCS/HO (***) (p<0.05).
Proliferation assays
The cell viability of hPDLs cultured onto root slices obturated with BioRoot RCS/HO, and AH-Plus/CWC technique after seeding for 3,7,14, and 21 days was evaluated by the WST-1 assay (Figure 3). The results demonstrated that the cell proliferation of experimental groups increased at 3,7,14, and 21 days after cultivation when compared to the controls, with no significant differences between groups by the 7th day. After 14 days of culture, the cell viability of hPDL cells cultured onto root slices treated with BioRoot RCS/HO has a significant difference over the slices treated with AH-Plus (p<0.05). After 21 days of analysis, both groups were significantly different than the control, and between them as well (p<0.05). These results showed that the filling materials didn't induce a cytotoxic effect on the hPDL cells, and rather improved the viability and the proliferation of cells.
Cell morphology (fluorescence)
Fluorescence analysis was performed to determine whether cells' growth, attachment, interaction with the material, and morphology. The fluorescence analysis showed that hPDL cells adhered and grew on the surface of the root model. The hPDL cells adhered to the root slices filled with BioRoot RCS/HO uniformly and covered all the slice surface showing a positive staining represented by a green apple color, and this was interpreted as adequate viability of the cells. The cells maintained a large, flat, elongated and spindle-shaped morphology, as shown in the fluorescence microphotographs (Figure 4).
Representative images of the hPDL cells seeded onto the root slices filling with AH-Plus/CWC are presented in Figure 5. The cells showed affinity to the experimental surfaces; isolated cells or cells in small disperse groups over the surface of the root slice were observed. Moreover, few spreading cells exhibited a flat shape (Figure 5).
The cell-material interaction analysis of the spreading and morphology of hPDL cells showed that the filling materials did not induce any visible changes in the cellular morphology, such as hydropic degeneration, granular to vacuolate cytoplasm or cell membrane rupture. Together, the results suggest that BioRoot RCS/HO and AH-Plus/CWC are both obturation techniques with low toxicity and indicate that the filling techniques favor the spread of hPDL cells, and support cell adhesion. The proliferation and adhesion assays demonstrated that hPDL cells adhered to a higher degree on the root slices obturated with BioRoot RCS/HO. The results also indicated that this group showed a better behavior for cell adhesion and proliferation than the AH-Plus/CWC.
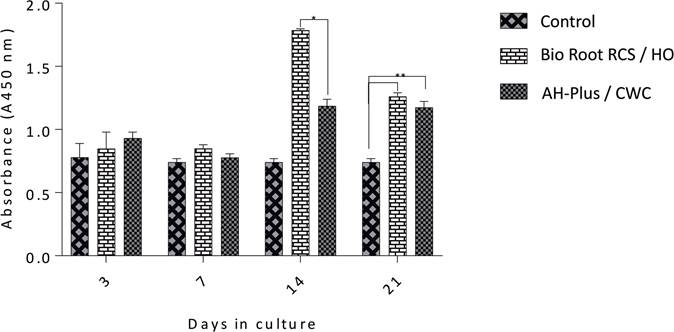
Figure 3 Results of the WST-1 assay. Cell proliferation of hPDL after 3,7,14, and 21 days in culture with Bio-Roo or AH-Plus. The difference in cell proliferation was statistically significant between AH-Plus/Continuous wave condensation (CWC) and BioRoot RCS/Hydraulic obturation (HO) by day 14 (*), and between control and BioRoot/HO and AH-Plus/CWC (**) by day 21 (p<0.05).
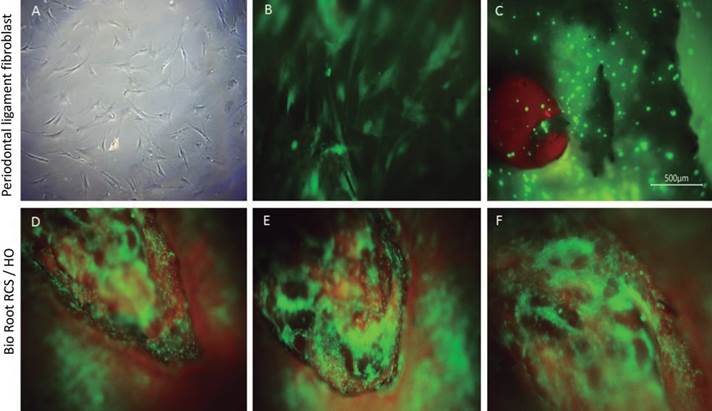
Figure 4 Microscopic evaluation of Human periodontal ligament cells (hPDLs) for group BioRoot RCS/HO after 24 hours. Representative images of hPDL cells' morphology seeded onto a tissue culture plate observed under an inverted microscope 4x (A). Incubation of hPDL cells with Cell-Tracker™ Green CMFDA after 30 minutes 20x (B). Fluorescence image of the cell-material interactions in the experiment corresponding to BioRoot RCS / HO (C,D,E,F). The hPDL cells onto control slice without any presence of the material (C). hPDL interaction over the surface of BioRoot that shows a biocompatible behavior whit the material (D,E,F).
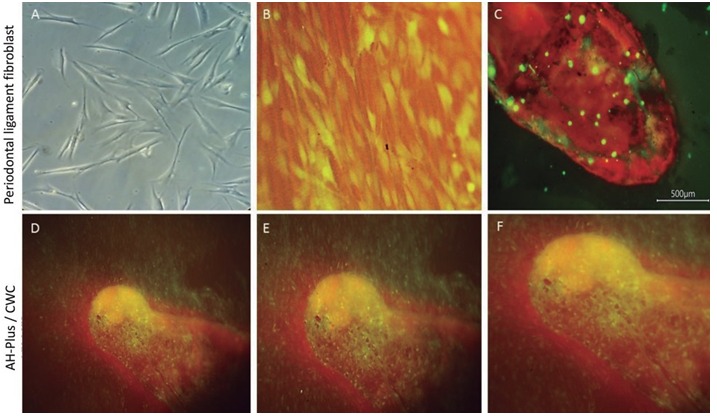
Figure 5 Microscopic evaluation of Human periodontal ligament cells (hPDLs) for group AH-plus CWC after 24 hours. Representative images of hPDL cells' morphology seeded onto a tissue culture plate observed under an inverted microscope 4x (A). Incubation of hPDL cells with Cell-Tracker™ Green CMFDA after 30 minutes (B). Fluorescence image of the cell-material interactions in the experiment corresponding to AH-Plus/CWC (C,D,E,F). The hPDL cells onto control slice without any material (C). The hPDL interaction over the surface of AH-Plus/CWC showed adhesion to the material, a uniform spreading pattern, and morphology similar to the control, interpreteted as a favorable biocompatibility whit the material.
Discussions
This study aimed to compare the adhesion and proliferation of hPDL cells in transverse sections of slice roots sealed with two different obturation techniques, hydraulic obturation with BioRoot RCS and continuous-wave condensation using AH-Plus. Several studies have investigated the degree of cytotoxicity of root canal sealers on hPDL cells (12,20,21), however, little information is available about the final response of each sealer once exposed to a certain obturation technique. The clinical rationale for investigating this cell response, is to evaluate the effect of possible extrusion of the obturation material from the root canal (i.e. when apical diameters are wider than expected), which may have the potential to induce a cytotoxic response in the surrounding periapical tissues (22). Few in vitro models evaluate cell cytotoxicity in similar conditions to those found in a clinical setting, as it was performed in the present study.
The biological evaluation was measured at different cell culture times for both obturation techniques. Despite their solubility, the di- and tricalcium silicate cement at the beginning of the setting process release ions of OH- y Ca2+, producing calcium hydroxide formation, contributing to their antimicrobial efficacy (23,24). Also, hydroxyapatite is formed on the materials' surface promoting their bioactivity. The results obtained from this study demonstrated that this effect influenced the values obtained in cell adhesion assays, which were higher in BioRoot RCS/HO than on the slices with AH-Plus/CWC. Nevertheless, when cell proliferation was evaluated, a similar response was observed within the first 7 days. By day 14, BioRoot RCS/HO root slides showed to increase the cell proliferation, and by day 21 both groups were different than the control, showing an increase response in BioRoot RCS/HO group. It was observed that after soaking the root slices in the culture medium, uniform sediment and granular material deposits were evident at the bottom of the culture plates that preserved the samples sealed with BioRoot RCS/HO. The sediments might represent solubility and loss of the material, characteristics previously reported for BioRoot RCS. However, in the present study, the BioRoot RCS/HO group had a better long-term stability, maintaining the cell growth onto the material. The improved response observed for the BioRoot RCS/HO root sections may indicate that although, solubility is expected, it may not be a biological impairment when the material offers acceptable biocompatibility.
Noteworthily, cell proliferation was higher in the experimental groups than in the controls after day seven. The available literature stated that all root canal sealers' toxicity reduces with time, regardless of the type of cement, and eventually, the sealers become an inert material (20,23,24,25). Most cytotoxicity studies performed with sealers have been carried out directly on the tabled-shaped material, which generally led to more significant limitations by not emulating its clinical use (25). In the present study, the manufacturer's protocol was followed, and the sealers were employed following the same parameters used in clinical practice. As seen in our results, the BioRoot RCS/HO group induced a faster proliferative response than the other groups.
In endodontic therapy, it is essential to assure a seal as hermetic as possible, since a three-dimensional obturation is essential to prevent re-infection and recontamination (i.e. preventing the passage of microorganisms and toxins to the periapical tissues) (26). It has been reported that sealing techniques by thermoplastification or CWC will adapt better to the complex root canal anatomy and improve density when compared to lateral condensation (27). Additionally, it has been argued that the continuous-wave technique seal grants a filling with fewer empty spaces than the hydraulic technique (28). However, this technique is not recommended for calcium silicate-based sealers, thus warm obturation techniques are mostly limited to other type of sealers such as epoxy resin based (29), with the exception of some calcium silicate sealers especially designed to be used with thermoplastic techniques. AH-Plus is a sealer that showed minor physicochemical changes after heat exposure (30). Hydraulic techniques, also described as a single cone technique performed with a bioceramic sealer, offers several biological advantages but are also perceived to be less effective due to the material's solubility, which could compromise the quality of the root canal treatment and reducing the hermetic sealing of the root canal. The present study showed that BioRoot RCS favored cell proliferation, and worked as a platform to improve cell biocompatibility, which eventually provided support to colonization and cell growth of periodontal fibroblast cells (31).
In this regard, the root canal treatment's success depends on the manipulation and selection of the filling material, depending on the clinical case to be treated. Endodontists must consider the disadvantages of sealant extrusion and choose an obturation technique depending on the initial diagnosis for individual cases and the outcome expected for each case.
The toxicity of BioRoot RCS and AH-Plus groups have been reported in a variety of experimental conditions with contradictory results (23,24,25,26,28,31,32). For each material, the viability and proliferation of hPDL cells increased and were superior to the control group in the present study. This study showed that both types of sealers allowed the growth of hPDL cells in vitro; thus, in the case of extrusion, periodontal tissues are expected to tolerate both cement types and techniques. Besides, BioRoot RCS and AH-Plus sealers exhibited excellent cytocompatibility after 24h in the cell adhesion assays, with a significant statis- tical difference when compared to the control; and the highest proliferation rate found in the BioRoot RCS root sections. Moreover, significant statistical differences in cell proliferation were observed after 14 days of culture between BioRoot RCS and AH-Plus. Previous research demonstrated that BioRoot RCS presents a higher solubility initially; then, the solubility decreased with time. As a result, these toxic substances' leaching decreased, consistent with the present study (23,24).
The low cytotoxicity of an endodontic sealer is one of the underlying conditions for a successful endodontic treatment and healing of the periodontium. Our results suggest that both materials offer acceptable biocompatible properties, allowing cell growth after 21 days of culture. Interestingly, AH-Plus demonstrated the best results after 21 days, which could be related to the cement's solubility and decreased toxic substance release by the sealant. In the evaluation of cell morphology, hPDL cells seeded onto BioRoot RCS showed a superior spreading pattern compared to those in contact with AH-Plus root sections. However, in both experimental groups, hPDL cells were able to adhere to the root surface and the obturation material.
Further in vitro and in vivo studies are required to confirm the suitability of calcium silicate-based utilizing a hydraulic technique and resin epoxy-based endodontic sealers with the continuous-wave technique for their clinical applications; and well-designed clinical trials are necessary to confirm the clinical impact of these techniques and materials.
Conclusions
The present in vitro study showed that BioRoot RCS/HO obturation is associated with a better biological response than AH-Plus/CWC and may well be regarded as a suitable alternative for endodontic treatments, improving its clinical relevance. The present in vitro results revealed that BioRoot RCS/HO and AH-Plus/CWC groups offers acceptable properties that modulate adhesion and proliferation of hPDL cells. However, to assess these findings in patients, clinical studies regarding their sealing properties also needed to evaluate clinical outcome for extended periods of time.
Stamen of funding
The present work was funded by the Natio- nal Autonomous University of Mexico grant number UNAM-DGAPA-PAPIIT-IA203522.
Disclosure statement of conflicts of interests
The authors have no conflict of interest to disclose.
Author contribution statement
Conceptualization and design: F.C.V.V. and L.Y.A.P.
Literature review: A.R.H.
Methodology and validation: F.C.V.V. and P.G.A.
Formal analysis: A.P.G.
Investigation and data collection: P.G.A.
Data analysis and interpretation: D.Ch.B.
Writing-original draft preparation: F.C.V.V. and D.Ch.B.
Writing-review & editing: M.M.A. Supervision: M.A.A.P.















