Introduction
Staining of dental structures is one of the most common clinical esthetic problems in dentistry. Bleaching procedures constitute a more conservative approach than other procedures, such as micro abrasion and restorative procedures.(1) Other dental restorative procedures such as diastema closure and the replacement of composite restorations may be required after bleaching. Studies have shown that the bond strength to enamel is reduced in composite restoration applications after bleaching (2,3).
Strategies aimed at eliminating the decreased bond strength include removal of the superficial enamel layer, treating the enamel with alcohol, use of adhesive-containing organic solvents to delay the bonding procedures, and the use of antioxidant agents (4,5,6). The general approach is to delay the bleaching procedures by from 24 hours to 2 weeks since waiting period is required before restoration to achieve normal bond strength values prior to bleaching (7,8). This situation entails risks such as microleakage, crown fracture, and secondary discoloration. These complications are undesirable for both the dentist and the patient (9).
Bleaching agents containing different concentrations of peroxide compounds are available for use in dental clinics. The residual oxygen-free radicals released by the application of peroxide compounds to the enamel surface penetrate the enamel structure. The presence of these radicals adversely affects the polymerization of the adhesive which applied during composite resin restorations and weakens the bond strength (10,11,12). Previous studies have reported that oxygen remains in the dental structure after bleaching and that residual oxygen may interfere with the polymerization reaction of adhesives (13,14,15).
One of the strategies recommended in recent years for eliminating the waiting phase while switching to restorative applications after bleaching is the use of antioxidant agents. However, it is still not clear which antioxidant agent is most successful. The aim of this in vitro study is to investigate the effectiveness of antioxidant agents and delayed bonding two weeks after office and home bleaching applications.
Materials and methods
Specimen preparation and bleaching application
Approval for this study was obtained from the Atatürk University Dental Faculty ethical committee, Turkey (08/2018). Debris was removed from 120 decay-and defect-free upper incisor teeth using pumice and rubber cups, and the teeth were stored in 0.2% thymol solution. Specimens were first cut from the cementoenamel junction and embedded in acrylic resin, after which they were stored in distilled water for 24 h before the assigned treatments were performed.
The labial surfaces of all specimens were polished using wet 400-and 600-grit silicon carbide papers to elicit flat homogeneous enamel surfaces without exposing the dentin. The specimens were randomly divided into two groups for office bleaching (hydrogen peroxide, 40% Opalescence Boost CP, Ultradent Products, USA) or home bleaching (Carbamide Peroxide, 10% Opalescence PF, Ultradent Products, USA) (Table 1). For the office bleaching application, the bleaching gels were applied at a 2-mm thickness to the top surface of each specimen following the manufacturer’s instructions. Following application, the bleaching gels were activated for 5 min with a LED light-curing unit (Valo LED, Ultradent Products, South Jordan, USA) in plasma emulation mode with an intensity setting of 3200 mW/cm². For the home bleaching application, the bleaching agent was applied to the specimens for 8 hours a day for 14 days. Except for during bleaching, the specimens were stored in distilled water. After the bleaching process, all specimens were washed with pressurized water, and the bleaching gel was removed.
Antioxidant application
The specimens in the home and office bleaching groups were subsequently divided into 24 subgroups. The first 12 groups (six office, six home) were kept in distilled water for 24 hours after whitening and were then exposed to antioxidant treatment (sodium ascorbate=SA, chitosan=C, Catalase=Ch, and epigallocatechin gallate=EGCG). Ten grams of SA powder was first dissolved in 100 ml distilled water to produce a 10% solution which was applied to the specimens for 10 minutes. The entire chitosan polymer used was first deacetylated to 95% using previously published methods (16). A 1% solution was prepared with acetic acid, mixed with 1% glucosamine, gently stirred until dissolved, and finally applied to the specimens for 10 minutes. The regimen for Ch (enzymatic activity units/mg protein 2000.00-5000.00 Hi-Media Labs Pvt. Ltd.) included application for 3 minutes. Specimens were then washed with deionized water and placed in vial containing artificial saliva. Ninety-five percent EGCG extract (Sigma-Aldrich Co., USA) was used to prepare a 1% concentration of EGCG including 100 μM through dilution in water-ethanol solution (1:1). The EGCG antioxidant solution was applied to each specimen for 10 minutes.
Thirty-seven percent phosphoric acid gel (Ultra Etch, Ultradent Products Inc., UT, USA) was applied to every specimen surface for 30 seconds, after which the specimens were rinsed for 15 seconds and dried for 10 seconds. The tooth surfaces were then restored using composite resin (Filtek™ Z250 XT, 3M, ESPE, USA) in line with the manufacturer's instructions. All specimens were polymerized for 40 seconds using a LED light-curing unit (Valo LED, Ultradent Products, South Jordan, UT, USA) in standard mode with an intensity of 1000 mW/cm². The light intensity of the curing unit was checked using a digital radiometer, and the light-curing unit was recalibrated for each group (Hilux Ultra Plus Curing Units, Benlioğlu Dental, Ankara, Turkey). The composite resin was applied to the negative control (N) group without any treatment (whitening or antioxidant), while positive control (P) specimens were subjected only to whitening procedures. The specimens
Statistical analysis
The data obtained as a result of the µTBS test and nanoleakage observation were recorded and statistical analysis was performed in SPSS 20.00 package program (SPSS Inc., Chicago, IL, USA). The Kolmogorov-Smirnov test was applied to confirm the normal distribution of the data. The µTBS data were analyzed with ANOVA, Duncan’s test and the paired t-test (α=0.05).
Table 1 Materials used in this study.
| Product | Contents | Manufacturer |
|---|---|---|
| Carbamide Peroxide (%10 Opalescence PF) | Glycerin, water, xylitol, carbamide peroxide, carbomer, PEG-300, sodium hydroxide, potassium nitrate, EDTA, sodiumfluoride. ph; 6.5 | Ultradent Poducts, South Jordan, UT, ABD |
| Hydrogen peroxide (% 40 Opalescence Boost CP) | Hydrogen peroxide, fluoride, potassium nitrate. ph: 6.4- 7.6 | Ultradent Poducts, South Jordan, UT, ABD |
| Sodium Ascorbate | C6H7NaO6 (powder) | Sigma-Aldrich, St. Louis, USA |
| Catalase | Obtained from beef liver | Sigma-Aldrich, St. Louis, USA |
| EGCG | 95% extract of EGCG | Sigma-Aldrich, St. Louis, USA |
| Chitosan | deacetylated to 95% | Sigma-Aldrich, St. Louis, USA |
| Adhesive (Singlebond universal) | MDP, phosphate monomer, HEMA, methacrylate-modified polyalkenoic acid copolymer, filler, ethanol, water, initiator, silane | 3M/ESPE, St. Paul, MN, USA |
| Composite Resin (Filtek Z250) | UDMA, Bis-EMA, TEGDMA and inorganic filler | 3M/ESPE, St. Paul, MN, USA |
EGCG: Epigallocatechin gallate, MDP: methacryloloxidesyl dihydrogen phosphate, HEMA: hydroxyethyl methacrylate, UDMA: Urethane dimethacrylate, Bis-EMA: Bisphenol A polyethylene glycol diether dimethacrylate, TEGDMA: Triethylene glycol dimethacrylate
Results
The µTBS test results showed that delayed bonding two weeks after bleaching increased the bond strength of bleached enamel, while the use of antioxidants effectively reversed the compromised bond strength of bleached enamel (p<0.05). Mean µTBS values were compared among all the groups, and a statistically significant difference was determined (p<0.05) (Table 2).
In the office bleaching groups, the mean µTBS values of the immediate antioxidant applied groups were statistically significantly higher than the P group (p<0.05). The highest mean µTBS values in the antioxidant groups were in the SA and C groups. Specimens’ mean µTBS values increased statistically significantly 14 days after bleaching, except for in the SA and Ch groups (p<0.05). No statistically significant difference was observed between the mean µTBS values of the P and SA groups among the specimens to which antioxidant was applied after 14 days (p>0.05). The highest mean µTBS value among the antioxidants was observed in the EGCG group 14 days after bleaching. The highest mean µTBS values in the N groups were observed in the immediately measured specimens and those measured after 14 days.
In the home bleaching group, no statistically significant difference was observed between the µTBS values of the groups to which antioxidant was applied immediately and the P group. However, the mean µTBS values of the antioxidant groups were higher than those of the P group. Antioxidant application after two weeks statistically significantly increased the bonding strength, except for in the EGCG group (p<0.05). The highest mean µTBS value among the antioxidants was observed in the C group in the specimens to which antioxidant was applied after 14 days. The highest mean µTBS values in the N groups were observed in the immediately measured specimens and those measured after 14 days.
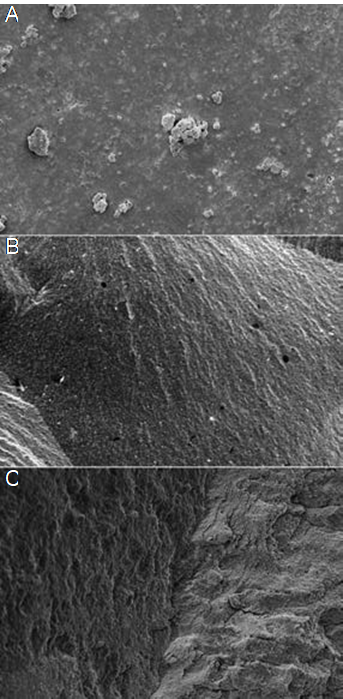
As a result of the failure analysis, adhesive type failure was observed more in the P and N groups of the specimens while the Mix type failure was observed more in the Ch groups (Table 3). Representative stereomicroscope and SEM micrographs of the failure surfaces of the specimens are shown in Figure 1 and Figure 2.
Table 2 The microtensile bond strength test results.
| Antioxidant application immediately after bleaching | - | - | Antioxidant application 14 days after bleaching | - | p** |
|---|---|---|---|---|---|
| Office (O) | P | 7.01±1.71ª | P | 10.37±3.14ª | 0.001 |
| - | N | 16.11±4.09ͨ | N | 18.89±4.39 ͩ | 0.022 |
| - | SA | 13.67±5.48ᵇ ͨ | SA | 13.93±3.85ªᵇ ͨ | 0.907 |
| - | C | 12.11±3.86ᵇ ͨ | C | 15.11±3.06ᵇ ͨ | 0.017 |
| - | EGCG | 11.96±4.84ᵇ | EGCG | 17.18±5.12 ͨ ͩ | 0.044 |
| Ch | 11.02±4.54ᵇ | Ch | 13.38±2.75ªᵇ | 0.061 | |
| p* | - | 0.001 | - | 0.001 | - |
| - | P | 8.15±2.80ª | P | 12.14±4.06ª | 0.035 |
| - | N | 15.73±3.76ᵇ | N | 24.67±3.49 ͩ | 0.001 |
| Home (H) | SA | 10.64±3.78ª | SA | 17.20±6.22ᵇ ͨ | 0.032 |
| - | C | 11.00±5.47ª | C | 18.73±3.25 ͨ | 0.003 |
| - | EGCG | 11.39±4.49ª | EGCG | 13.88±5.80ªᵇ | 0.076 |
| - | Ch | 9.99±2.20ª | Ch | 14.00±3.69ªᵇ | 0.007 |
| p* | - | 0.003 | - | 0.001 | - |
Different lower case letters in the columns show statistical difference (One way ANOVA). p* Comparisons in rows; pair t test. p**
O: Office, H: Home, P: Positive control, N: Negative control, SA: Sodium Ascorbate, C: Catalase, EGCG: Epigallocatechin gallate, Ch: Chitosan.
Table 3 Number (%) of fracture types in μ TBS specimens as analyzed by stereo-microscopy.
| Failure Type (%) | - | - | - | - | - | - | - | - |
|---|---|---|---|---|---|---|---|---|
| Groups | Antioxidant application immediately after bleaching | - | - | - | Antioxidant application 14 days after bleaching | - | - | - |
| - | Office | - | Home | - | Office | - | Home | - |
| P | Adhesive | 74 | Adhesive | 76 | Adhesive | 93 | Adhesive | 90 |
| - | Cohesive | - | Cohesive | - | Cohesive | - | Cohesive | 3 |
| - | Mix | 26 | Mix | 24 | Mix | 17 | Mix | 6 |
| N | Adhesive | 60 | Adhesive | 66 | Adhesive | 66 | Adhesive | 51 |
| - | Cohesive | 20 | Cohesive | 17 | Cohesive | 7 | Cohesive | 16 |
| - | Mix | 20 | Mix | 17 | Mix | 27 | Mix | 33 |
| SA | Adhesive | 48 | Adhesive | 26 | Adhesive | 40 | Adhesive | 33 |
| - | Cohesive | 26 | Cohesive | 34 | Cohesive | 10 | Cohesive | 26 |
| - | Mix | 26 | Mix | 40 | Mix | 50 | Mix | 41 |
| C | Adhesive | 20 | Adhesive | 40 | Adhesive | 13 | Adhesive | 24 |
| - | Cohesive | 16 | Cohesive | 26 | Cohesive | 23 | Cohesive | 6 |
| - | Mix | 63 | Mix | 34 | Mix | 63 | Mix | 70 |
| EGCG | Adhesive | 13 | Adhesive | 33 | Adhesive | 11 | Adhesive | 16 |
| - | Cohesive | 43 | Cohesive | 36 | Cohesive | 53 | Cohesive | 43 |
| - | Mix | 43 | Mix | 31 | Mix | 36 | Mix | 41 |
| Ch | Adhesive | 7 | Adhesive | 14 | Adhesive | 7 | Adhesive | 14 |
| - | Cohesive | 33 | Cohesive | 36 | Cohesive | 43 | Cohesive | 33 |
| - | Mix | 60 | Mix | 50 | Mix | 50 | Mix | 53 |
O: Office, H: Home, P: Positive control, N: Negative control, SA: Sodium Ascorbate, C: Catalase, EGCG: Epigallocatechin gallate, Ch: Chitosan.
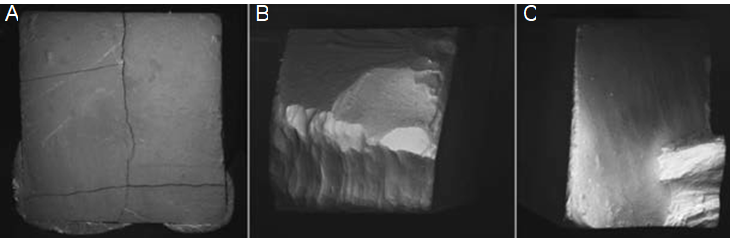
Figure 1 Representative stereomicroscope (X50) photomicrographs of failure surfaces of specimens. A: Adhesive type failure, B: Cohesive type failure in dentin, C: Mixed type failure.
Discussion
The bleaching procedure destabilizes the hybrid layer in the region of the junction between resin and tooth tissues and adversely affects the bond strength of the resin (17). Reactive oxygen, which penetrates into the tooth structures after bleaching, both prevents the penetration of the resin and weakens its polymerization (18). SEM examinations of the teeth restored after bleaching have revealed decreased resin tag formation of the resin tag decreased and an irregular structure in the hybrid layer due to this mechanism. Bonding failures were detected in composite resin restorations performed immediately after bleaching treatments in these studies (19,20). The initial decrease in enamel bond strength after bleaching is therefore of clinical significance and represents a problem to be overcome. Although studies have investigated means of increasing the bond strength of composite resin restoration after bleaching, no definitive protocol has yet emerged (8). The purpose of the present study is to contribute to the current literature by examining the effect of different antioxidants on bond strength in bleached enamel. In agreement with the previous literature, bleaching application in this study reduced mean µTBS values in all specimens.
Studies have recommended waiting times between 24 hours and 4 weeks in order to increase post-bleaching bond strength (3). However, such delays may not be possible in cases that require early treatment after whitening. In such cases, it is recommended that antioxidants such as sodium ascorbate, catalase, acetone, ascorbic acid, ethanol, hydroxyanisole and sodium bicarbonate be applied to the whitened tooth surfaces (21,22). In the present study, sodium ascorbate, catalase, chitosan, and ECGC antioxidants were applied to bleaching teeth immediately and after two weeks, and the bond strength of the specimens was measured and compared with that of the control groups. Previous research has shown that by applying 10% sodium ascorbate gel for sixty minutes, the bonding strength to enamel may be increased (23). However, this application period is thought to be quite long for the clinician and the patient. For this reason, it has been suggested to investigate the effect of applying 10% sodium ascorbate gel in shorter periods after bleaching with 40% hydrogen peroxide on enamel bonding (15).
No statistically significant difference was determined in the present study between the mean µTBS values of the specimens to which SA was applied for 10 minutes immediately after bleaching with 40% hydrogen peroxide and the N group. The immediate bonding strength was restored in the specimens to which SA antioxidant was applied. A similar situation applies to the C group in specimens exposed to antioxidants immediately after bleaching. The natural antioxidant SA, with its significant biocompatibility, is one of the most studied antioxidants (24). Miranda et al. (25) and Wang et al. (26) reported similar bond strength values to non-bleached specimens by applying SA to the enamel surfaces after bleaching. In the present study, the mean µTBS value ın the SA groups was statistically significantly lower than in the N groups, apart from in the groups in which application occurred immediately after office bleaching, although the mean µTBS value was higher than the P groups. Similarly, Abraham et al. (27) reported an increased µTBS value in specimens exposed to SA, although the mean µTBS value in these specimens was not as high as that in the non-bleached groups. In the present study, sufficient bond strength values were not achieved in the groups exposed to immediate ECGC and Ch antioxidants, although the mean µTBS values increased by applying these antioxidants to the specimens after two weeks of storage in artificial saliva.
The acidic properties of carbamide peroxide and the end products and residual oxygen formed by breaking down during the bleaching process have been reported to affect the enamel crystals and to lead to demineralization (28). Due to these properties, it was reported that the Decreased bond strength as a result of bleaching with carbamide peroxide has also been reported due to these properties (29,30). In the present study, the mean µTBS value of all specimens decreased as a result of home bleaching with 15% carbamide peroxide. Similarly to the office bleaching groups, the mean µTBS values of the home bleaching groups increased with the application of antioxidants either immediately or after 2 weeks. Consistent with the present study, Güler et al. (24) found that bond strength increased with the use of SA and other antioxidants in specimens to which 16% carbamide peroxide was applied. Thakur et al. (31) reported a significant increase in binding strength following the application of antioxidants to specimens treated with carbamide peroxide. No significant difference was observed in the present study between the mean µTBS values of the immediate Ch group and the P group (no antioxidant) for home bleaching. However, the mean µTBS value of the specimens exposed to Ch after 2 weeks increased significantly. This may be due to these specimens being stored in artificial saliva for 2 weeks. The increase in bond strength achieved by storing home bleaching specimens in artificial saliva was only not statistically significant in the ECGC groups. EGCG has been reported to be capable of halting the activation of pro-matrix metalloproteinase (MMP)-2, MMP-2, and MMP-9 (32). These MMPs inhibit collagenase activity, thus preventing collagen denaturation (33). Collagen occupies an important place in the connection of resin tags in hybrid layer formation (34). These properties of EGCGs may provide protection for the hybrid layer and increase the bond strength, thus suppressing collagen degradation.
One of the effective factors in reducing the bonding strength to enamel after bleaching is the weakening of the enamel organic component and mineral structure (35). One proposed solution to this problem involves the postponement of composite resin application after bleaching. In the presence of saliva, remineralization takes place on the enamel surfaces, and inorganic components moving away from the enamel surface are reconnected to the surface and act as oxidizers (36). Lago et al. (37) obtained similar bond strength values to those of non-bleaching specimens by keeping specimens in artificial saliva for 2 weeks after bleaching. In the present study, increases in mean µTBS values were achieved in all groups by keeping the specimens in artificial saliva for 2 weeks. However, no significant difference was found between the specimens exposed to antioxidants immediately and after 2 weeks in the SA and Ch office bleaching groups and EGCG home bleaching groups. In addition, the mean µTBS values of the specimens exposed to antioxidant application after 2 weeks of storage in artificial saliva were lower than those in the N groups. Our results indicate that the mean bond strength values of the specimens exposed to antioxidants and/or stored in artificial saliva were not achieved in the N groups.
Conclusion
Within the limitations of this in vitro study, it may be concluded that;
The use of antioxidants following office bleaching created a significant increase in the bond strength values of composite resin to enamel (p<0.05).
The use of antioxidants following home bleaching did not significantly increase the bond strength values (p> 0.05).
Significant increase in the bond strength of holding & antioxidant use in office bleaching samples was observed in Catalase and EGCG groups; (p <0.05).
The positive effect of holding & antioxidant use on bond strength was observed in Sodium Ascorbate, Catalase and Chitosan groups in home bleaching samples (p<0.05).
The use of different antioxidants before bonding procedures on bleached enamel may significantly neutralize the negative effects of bleaching agents and increase the bond strength. The effects of the use of different antioxidants on the bond strength of bleached enamel should now be examined, and our results should be supported by clinical studies.