Introduction
Tomato (Solanum lycopersicum) is known as a self-pollinating plant (Lin et al., 2014). Similar to other self-pollinating plants, selection and progeny testing are generally conducted in the early generations (Acharya et al., 2018; Cappetta et al., 2020; Kahani & Hittalmani, 2016). This early generation testing helps improve breeding efficiency by selecting superior genotypes and eliminating inferior ones from the segregating populations (Abdelmoghny, 2021; Crossa et al., 2017; Dama et al., 2022; Limbongan et al., 2021; Ramos Guimarães et al., 2021; Wibisono et al., 2022). However, due to environmental effects, sometimes the selected genotypes do not exhibit optimal performance in later generations, especially regarding yield characteristics (Avdikos et al., 2021; Collard et al., 2017; Wibisono et al., 2019; Wibisono et al., 2021).
In plant breeding, the F2 to F6 generations are critical phases for selection and evaluation (Ahmad et al., 2017; Pontes Júnior et al., 2016; Yadav et al., 2021). Variance and heritability are mostly assessed in F2 to F6 generations to predict the response of the selected population (Hakim & Suyamto, 2017; Oliveira Silva et al., 2021). Selection conducted over several generations with the same intensity has been reported to reduce the values of heritability, phenotypic variance, and selection response (Asrat, 2021; Sinha et al., 2021).
Several studies related to the selection response of yield component traits in self-pollinated plants have show different patterns for each species (de Paula et al., 2020; Purnamasari et al., 2019; Ritonga, Chozin et al., 2018). In Capsicum annuum L., the selection response observed fron the F2 to F6 generations exhibited a decreasing pattern in each generation (Ritonga, Syukur et al., 2018). However, in different crossbred populations, it progressed in an increasing pattern (Rosminah et al., 2019). Selection responses within the same generation range for yield component traits of Vigna unguiculata L. showed an irregular pattern but a lower mean value in F6 compared to its F4 generation (Varghese et al., 2021).
The selection response that tends to decrease in later generations is not only caused by genetic factors from the parental lines but also external factors such as environmental influences and selection methods (Wanga et al., 2021). In self-pollinated crops such as rice (Collard et al., 2017; Rini et al., 2018), tomato (Hernández-Leal et al., 2019), and chili pepper (Massot Padilha & Barbieri, 2016), pedigree selection from early generations have been carried out to improve generative traits (Hamam, 2014; Sarutayophat & Nualsri, 2010) as well as resistance to biotic stress (Presello et al., 2005).
Pedigree selection from early generations aims to maintain superior segregation and leverage the natural potential of self-pollinating plants in assembling pure breeds (Avdikos et al., 2021; Thien Tran et al., 2021). However, when performance in early generations is controlled by epistatic genes, pedigree selection is not recommended (Khalaf et al., 2021). In this case, the superiority of the segregants is due to non-allelic interactions (epistasis), while there is no such interaction in the subsequent generations in self-pollinated plants (Fisher, 1919; Khalaf et al., 2021). This study was conducted to analyze the performance and yield components of several tomato populations using the pedigree method in lowland environments, Bogor, West Java, Indonesia.
Materials and methods
Study area
This study was conducted from December 2018 to April 2019, encompassing the selection and development of F2 to F6 populations at the Experimental Garden of Bogor Agricultural University, Tajur II (207 meters above sea level), Bogor, West Java, Indonesia. The average environmental temperature average at Tajur II during December 2018-April 2019 was 26.03 °C, based on the data from West Java Climatology Station, Bogor, Indonesia.
Plant materials
Seeds of tomato (Solanum lycopersicum) were obtained from the Plant Breeding Laboratory, Department of Agronomy and Horticulture, Bogor Agricultural University. Four generations resulting from the 99D×Tora cross were utilized in the study, comprising 200 plants of the F2 generation and 100 plants of each of the F3, F5, and F6 generations, respectively. The 99D variety is a pure line of tomato with large fruit size, developed in highland areas, (>600 meters above sea level). Tora, on the other hand, is an open-pollinated (OP) variety with medium-sized fruit suitable for cultivation in medium (300 - 600 m a.s.l.) to lowland (0 - 300 m a.s.l.) areas. The crossbreeding was aimed at obtaining recombinants or lines with large fruit size while being adaptable to lowland areas. Intan and Ratna varieties (open-pollinated (OP) varieties or non-hybrids) served as control varieties, which are commercially available varieties. The use of commercial varieties adheres to the regulations for variety testing and release in Indonesia. The reason for not using 99D and Tora directly is that they are proprietary pure lines owned by the company (PT BISI) and are not permitted for planting outside the company. Aditionally, Tajur is not owned by PT BISI.
Procedures
The entire experimental population was planted in the same season and field. Planting took place in open fields using a double-row system with two rows per single bed. Rows were spaced 0.5×0.6 m apart, and beds were 1 m apart. Each bed measured 20×1 m, accommodating 80 plants per bed. Seven days before planting, each bed received 15 kg of manure, 2 kg of urea and KCl, 3 kg of SP-36 (a phosphate and sulfate fertilizer containing 36 % phosphorus as P2O5), and 4 kg of dolomite. Black silver mulch was applied to cover each bed.
Plant maintenance included irrigation, fertilizer application, as well as pest and disease control. A NPK (16:16:16) fertilizer solution at a concentration of 15 g L-1 was applied at 2, 3, 4, and 5 weeks after planting (wap). Supplementary fertilizer was applied by pouring 250 mL of solution for each plant into a hole located 8-10 cm from the base of the plant stem. Gandasil D and Gandasil B fertilizers, foliar fertilizaers, were applied once a week along with spesticide praying according to the plant growth phase, with a concentration of 2 g L-1 from 2 to 5 weeks after planting. Gandasil D contains 14 % nitrogen, 12 % phosphate, 14 % potassium, 1 % magnesium and other micronutrients to promote vegetative growth. Gandasil B contains 16 % nitrogen, 20 %, phosphorus, 30 % potassium, and 3 % magnesium to promote fruiting or flowering. Weed control was performed manually.
Observations were made on quantitative yield components traits of all individual plants (Mawasid et al., 2019). The observed traits included flowering time (days after planting, dap), harvest time (dap), fruit length (cm), fruit diameter (cm), fruit weight (g), fruit weight per plant (g), and number of fruits. Ten fruit samples were measured, based on the mean measurement. Typically, the samples used for measurement were obtained from the early stages of harvest.
Data analysis
The analysis aimed to understand the pattern of selection responses across several generations. It included teo main components: 1) genetic advance to determine the difference in the mean value between selected population and the base population, as described by Syukur et al. (2015) (equation 1), and 2) estimation of narrow-sense heritability using offspring-parent regression with two segregated populations (F3 and F2, etc), following Falconer & Mackay (1996) (equation 2).
G = Fn - Fn-1 (1)
Where G represents the genetic advance, Fn is the mean of Fn population, and Fn-1 is the mean of Fn-1 population.

Where h2ns denotes the narrow-sense heritability, bOP is the offspring-parent regression, CovOP represents the variance between offspring and parent, and  is the variance of the F2 population. Heritability is classified as low (< 20 %), moderate (20 % ≥ h2ns ≥ 50 %), and high (> 50 %), according to Syukur et al. (2015).
is the variance of the F2 population. Heritability is classified as low (< 20 %), moderate (20 % ≥ h2ns ≥ 50 %), and high (> 50 %), according to Syukur et al. (2015).
Results
Selection response of several generations
The mean values of each generation and the comparison for each observed trait are presented in Table 1. A distinct selection was observed for each trait. Flowering time increased from the F2 to F3 generations but decreased from the F5 to F6 generations. Similarly, harvest time exhibited a similar trend. Flowering (Figure 1a) and harvest time (Figure 1b) shifted towards the left side of the graph, indicating improved traits for both flowering and harvest time, as they are correlated with early maturity.
Table 1 Mean ± standard deviation of tomato (Solanum lycopersicum) yield components in F2 - F6 generations of 99D×Tora compared to Intan and Ratna varieties. Bogor Agricultural University, Bogor, Indonesia. 2018-2019.
| Ch | Mean | |||||
| F2 | F3 | F5 | F6 | Intan | Ratna | |
| FT | 30.74±2.41bc | 29.27±2.72b | 29.39±2.07b | 31.53±3.40c | 27.82±3.42a | 30.13±4.05bc |
| HT | 70.69±3.14b | 70.43±3.57b | 71.33±4.11b | 75.22±3.42c | 68.18±3.21a | 70.00±3.54b |
| FL | 6.56±0.70c | 6.77±0.68c | 6.15±0.63b | 5.90±0.47b | 4.44±0.65a | 4.50±0.55a |
| FD | 4.79±0.62a | 4.87±0.56a | 4.85±0.48a | 4.68±0.42a | 4.88±0.58a | 5.48±0.85b |
| FW | 75.13±19.80b | 79.88±20.40b | 76.16±17.16b | 58.37±17.07b | 58.37±15.93a | 52.36±17.23a |
| FWP | 1627.27±824.04b | 1697.80±691.50b | 1745.39±746.89b | 1156.45±541.64b | 1156.45±446.23a | 1168.25±468.37a |
| NF | 40.01±16.45bc | 44.45±18.09c | 38.92±12.60bc | 32.78±9.39ab | 31.45±9.60a | 35.13±12.93ab |
Remarks: values marked with different letters in the same row show significant differences based on Duncan Multiple Range Test at p<0.05. Ch: characters, FT: flowering time (days after planting, dap), HT: harvest time (days after planting, dap), FL: fruit length (cm), FD: fruit diameter (cm), FW: fruit weight (g), FWP: fruit weight per plant (g), NF: number of fruits. / Observaciones: los valores marcados con letras diferentes en la misma fila muestran diferencias significativas basadas en la Prueba de Rango Múltiple de Duncan en p<0,05. Ch: caracteres, FT: época de floración (días después de la siembra, ddp), HT: época de cosecha (días después de la siembra, ddp), FL: longitud del fruto (cm), FD: diámetro del fruto (cm), FW: peso del fruto (g), FWP: peso de frutos por planta (g), NF: número de frutos.

Figure 1 Selection response in F2 - F6 generations of 99D×Tora tomato (Solanum lycopersicum). a. Flowering time (days after planting, dap); b. Harvest time (days after planting, dap). Bogor Agricultural University, Bogor, Indonesia. 2018-2019.
Fruit length, fruit diameter, fruit weight, and number of fruits also showed a positive selection response in F2 to F3 generations, and a decreased in F5 and F6 generations. Fruit weight per plant showed a genetic advancement in the mean value fromo F2 to F3 generation, and in F5 generation, the mean fruit weight was still higher compared with the F3 population. The decrease in fruit weight per plant was only seen in the F6 generation. Although the F6 generation had a lower mean value than F2 for each character, its fruit weight and fruit weight per plant characters were still higher than those of the Intan and Ratna varieties. Fruit weight and fruit weight per plant are the main yield component characters.
The mean and variance of flowering time fluctuated among generations (Figure 1a), as did harvest time (Figure 1b). The mean value of flowering time increased the F3 generation but gradually decreased in F5 to F6 generations. As for variance (Table 2), its value showed an increase in F3 compared with the F2 generation, a decrease in F5, and followed by another increase in the F6 generation. The mean value of harvest time showed similar advancement to the flowering time character, but not its variance. For traits with positive values on the left side (Table 1), such as flowering time, a lower value indicates an improvement. In other words, when the value is low, it is considered to have increased. Similarly, the opposite applies. The harvest time’s variance increased gradually in the F3 and F5 generations but decreased in the F6. The changes of the mean value among generations can be inferred from the curve’s peak position to the X-axis, while the value of variance can be seen from the curve’s peak position to the Y-axis. A higher peak reflects lower variance.
Table 2 Variance of tomato (Solanum lycopersicum) yield components in F2 - F6 generations of 99D×Tora compared to Intan and Ratna varieties. Bogor Agricultural University, Bogor, Indonesia. 2018-2019.
| Ch | Variance | |||||
| F2 | F3 | F5 | F6 | Intan | Ratna | |
| FT | 5.83 | 7.41 | 4.30 | 11.59 | 11.76 | 16.41 |
| HT | 9.87 | 12.75 | 16.97 | 11.76 | 10.36 | 12.57 |
| FL | 0.57 | 0.47 | 0.40 | 0.22 | 0.43 | 0.31 |
| FD | 0.39 | 0.32 | 0.24 | 0.18 | 0.34 | 0.73 |
| FW | 392.04 | 415.96 | 294.33 | 291.50 | 253.85 | 296.78 |
| FWP | 679038.13 | 478174.67 | 557838.02 | 293371.40 | 199122.27 | 219371.64 |
| NF | 270.62 | 327.13 | 158.65 | 88.22 | 92.07 | 167.27 |
Remarks: values marked with different letters in the same row show significant differences based on Duncan Multiple Range Test at p<0.05. Ch: characters, FT: flowering time (days after planting, dap), HT: harvest time (days after planting, dap), FL: fruit length (cm), FD: fruit diameter (cm), FW: fruit weight (g), FWP: fruit weight per plant (g), NF: number of fruits. / Observaciones: los valores marcados con letras diferentes en la misma fila muestran diferencias significativas basadas en la Prueba de Rango Múltiple de Duncan en p<0,05. Ch: caracteres, FT: época de floración (días después de la siembra, ddp), HT: época de cosecha (días después de la siembra, ddp), FL: longitud del fruto (cm), FD: diámetro del fruto (cm), FW: peso del fruto (g), FWP: peso de frutos por planta (g), NF: número de frutos.
Selection responses of fruit length and fruit diameter (Figures 2a and 2b, respectively) showed an increase in the mean value in the F3 generation and subsequent decrease in F5 to F6. Similarly, fruit weight and the number of fruits (Figures 3a and 3b) exhibited an increase in the mean value in the F3 generation, followed by a decrease from F3 to F6. Variances of fruit length and fruit diameter decreased gradually from F3 to F6, while variances of fruit weight and the number of fruits increased in F3 but gradually decreased from F5 to F6 generations.
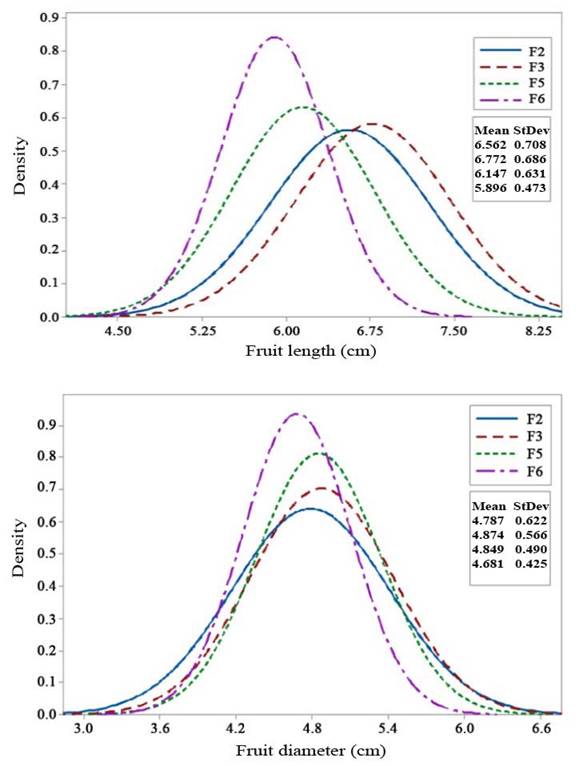
Figure 2 Selection response in F2 - F6 generations of 99D×Tora tomato (Solanum lycopersicum). a. Fruit length (cm); b. Fruit diameter (cm). Bogor Agricultural University, Bogor, Indonesia. 2018-2019.
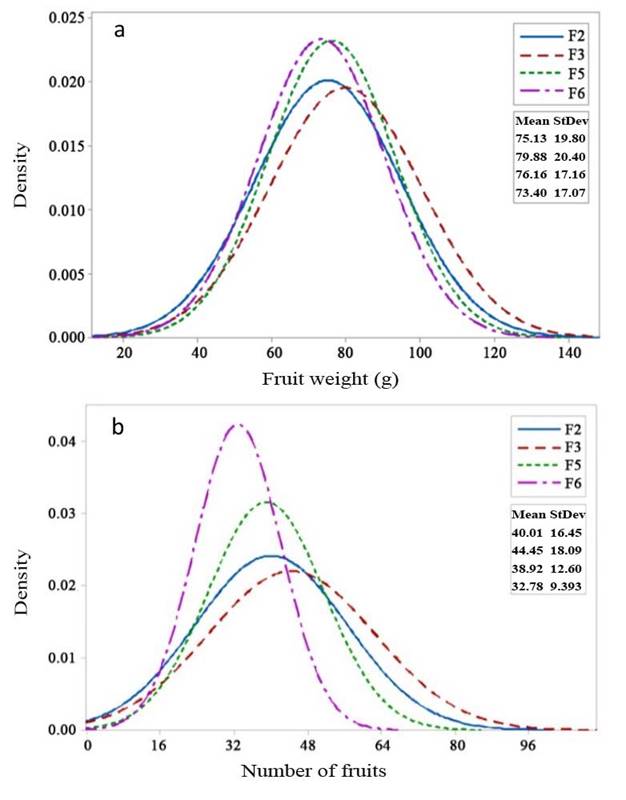
Figure 3 Selection response in F2 - F6 generations of 99D×Tora tomato (Solanum lycopersicum). a. Fruit weight (g); b. Number of fruits. Bogor Agricultural University, Bogor, Indonesia. 2018-2019.
Selection response of fruit weight per plant was shown in Figure 4. The mean value of this character increased in F3 to F5, and decreased in F6 generation. On the other hand, variance of fruit weight per plant showed a gradual decrease from F3 to F6 generation.
Heritability
The narrow-sense heritability values between early (F2 - F3) and later (F5 - F6) generations are shown in Table 3. Heritability in the early generation was greater than in the following generations, suggesting a decrease in heritability along with the fixation process.
Table 3 Narrow-sense heritability values between F2 - F3 and F5 - F6 generations of 99D×Tora tomato (Solanum lycopersicum). Bogor Agricultural University, Bogor, Indonesia. 2018-2019.
| No | Characters | h2 ns (%) | |
| F2 - F3 | F5 - F6 | ||
| 1 | Flowering time (dap) | -16.07 (low) | -23.47 (low) |
| 2 | Harvest time (dap) | -20.61 (low) | -10.33 (low) |
| 3 | Fruit length (cm) | 1.43 (low) | 1.29 (low) |
| 4 | Fruit diameter (cm) | 2.63 (low) | 1.64 (low) |
| 5 | Fruit weight (g) | -1.82 (low) | -11.52 (low) |
| 6 | Fruit weight per plant (g) | 8.62 (low) | 5.57 (low) |
| 7 | Number of fruits | 13.12 (low) | 11.33 (low) |
Remarks: h2ns: narrow-sense heritability. / Observaciones: h2ns: heredabilidad en sentido estricto.
The highest heritability in the early generation was observed in the number of fruits (13.12 %) character, while the lowest was found in harvest time (-20.61 %). The character with the highest heritability in the next generation was number of fruits (11.33 %), and the lowest was flowering time (-23.47 %).
Discussion
The selection response was observed by comparing the mean of one generation (Fn) with the subsequent generation (Fn+1). There was a difference in the selection method used in this experiment between F2 and F3 populations, as well as the F4 to F6 populations. The F2 and F3 populations were selected using a weighted index selection method from the previous study (Mawasid et al., 2019), while the F4 to F6 populations were selected based on visual observations (eyeballing) of different lineages, and the genotype with the best performance was chosen. The F5 and F6 populations reflected the selection response of the later generations derived from pedigree selection of the F3 population from the previous experiments (Mawasid et al., 2019).
The F4 population was not included in the planting due to a decrease in its seed vigor. The F5 and F6 populations could be used as simulators since both were obtained from the 99D×Tora cross (parental) and selected using the pedigree method. The genetic constitutions of the F5 and F6 generations would be similar to those of the F2 and F3 populations. Intan and Ratna varieties were used as comparisons. These two varieties were of pure breed, registered, and commercialized.
In general, there was a positive selection response in the early generations but a negative response in the later generations. The same response to tomato yield components was also reported by Ahmad et al. (2017) and Ahmad et al. (2018). This could be due to differences in the inheritance pathways used or the dominance of epistasis gene action due to the existing environmental stress (Mawasid et al., 2019). Epistasis genes seemed to be the main cause, considering that the F2 to F3 and F4 to F6 generations were obtained from pedigree selection, where only the best genotypes would be continued. There is a decrease in the variance in each generation because pedigree selection leads to the formation of a homozygous homogeneous population (Acquaah, 2012; Syukur et al., 2015).
The value of variance changed in each observed generation. A pedigree-derived generation did not always have a lower value than its previous generation (Table 2). This happened because selection was carried out on several characters at once through index selection. There was a possibility of uneven fixation on all characters. Selection carried out on a character will encourage fixation on its controlling genes, but when selected individuals have higher heterozygosity, segregation will occur which causes the variance of the later generation to increase. Inaccuracy in selection can occur when the proportion of non-additive genes (dominant and epistatic) in a character are greater than the additive genes. A smaller variance reflects the fixation of its genotypes and an increase in its homozygosity. If the variance is already low and the phenotype is good (yield, etc.), then it is worthy of multi-location testing to be released as an open-pollinated variety.
In general, selection responses of flowering time and harvest time from F2 to F6 moved to the right. Fruit length, fruit diameter, fruit weight, fruit weight per plant, and number of fruits moved to the left, which meant a negative selection response, especially in the later generation. Pedigree selection in this study resulted in the lowest mean and variance in F6 generation. This indicated that the gene fixation process in the early generations eliminated superior traits. As previously explained, in an environmentally stressed condition, the action of non-allelic genes (epistasis) plays a major role. In the later generations, the action of these genes will be eliminated because they are not fixed (Mather & Jinks, 1982), so that the superiority of the genotype in the early generations will disappear.
Based on the data presented in this paper, pedigree selection was not suitable for selection in a stressed condition, especially in the lowland. This was due to the possibility of inaccuracy selection in early generations. Selection using bulk or single seed descent is more recommended (Mistry et al., 2016; Said, 2014), considering that variance among generations is still controlled until later generations. Selection will be more convincing because performance and variance within a population are controlled by the genotype which has been fixed.
Narrow-sense heritability is defined as the fraction of phenotypic variance that can be attributed to variation in the additive effects of genes. A negative heritability value indicated that variances in these characters were not statistically inherited in the next generations, or in other words, h2ns = 0. The low heritability of each character can be caused by environmental factor and non-additive genes (Mawasid et al., 2019). The selection process applied to each generation will cause changes in gene frequency so that it will affect the heritability values of the next generation. Heritability values decreased in the next generations due to gene fixation and increased homozygosity. A decrease in heritability value in F3 to F5 generations was also reported by Ahmad (2016) for the yield component of Vicia faba L.
Conclusions
Selection across multiple generations tends to decrease the variance and heritability of characters. This reduction in value occurs due to fixation, which alters gene frequency and increases homozygosity. While the mean value tends to increase in the early generations, it often decreases in later ones due to the fixation process, which eliminates the influence of epistatic genes. Therefore, pedigree selection in the early generation may not be suitable for selection in the lowland conditions. Instead, the bulk or single seed descent method are recommended because they were capable of maintaining variance in later generation.
















