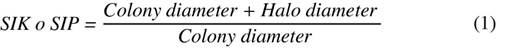Introduction
The increase in agricultural practices in recent years has generated a decrease in soil organic matter. The availability of nutrients such as phosphorus (P) and potassium (K) is essential for plant growth and development (Alavaisha et al., 2019). In order to cover these nutritional needs, farmers applied mineral fertilization such as mica and phosphoric rock. However, 80 % of these minerals are not transformed into bioavailable compounds due to the absence of solubilizing microorganisms, which makes it an inefficient practice (Osorno Bedoya & Osorio Vega, 2017). Another alternative is the application of large amounts of chemical fertilizers, which, in the long term, affects microbial biodiversity, micronutrient imbalance, acidification, and soil degradation (Kumar Bhatt et al., 2019).
A biotechnological alternative to mitigate these problems is the implementation of mountain microorganism (MM) bioferments, a technique that consists of taking leaf litter from native soils not treated with agrochemicals, with a high accumulation of organic matter and carrying out a fermentative process to enhance the beneficial microbiota in which, a great diversity of genera of yeasts, molds, and bacteria such as Saccharomyces, Pichia, Trichoderma, Penicillium, Aspergillus, Azotobacter, Azospirillum, Rhizobium, Pseudomonas, Bacillus, and Streptomyces can be found (Campo-Martínez et al., 2014; Quiñones-Ramírez et al., 2016). These microorganisms develop multiple capacities such as the secretion of beneficial substances such as indoleacetic acid (IAA), a hormone that promotes plant growth, killer proteins and antibiotics that control plant pathogens. soil, chitinases, phytases, and organic acids that promote plant nutrition and bioavailability of minerals, among others (Spadaro & Droby, 2016; Umaña et al., 2017).
Among the microorganisms registered and present in the MM bioferments, yeasts stand out due to their large size, their rapid growth, and the nutritional activity that allows them to adapt and activate their biochemical machinery for the production of different phytohormones such as auxins, cytokinins or gibberellins, enzymes that contribute to the solubilization of nutrients and antimicrobial compounds such as panomycocin and zymocin, acting as biostimulants and biocontrollers (Altınbay Izgu et al., 2011; Nutaratat et al., 2016; Tzelepis & Karlsson, 2019). However, there is little evidence to demonstrate these agronomic potentialities of yeasts present in MM, as supported by Hernández-Fernández et al. (2021), especially as efficient microorganisms with more than one agronomic capacity of interest. For this reason, the objective of this study was to evaluate, at in vitro level, the biofertilizing capacity and growth-promoting capacity of antagonist yeasts isolated from MM bioferments.
Materials and methods
Location
The experiments were carried out from March to December of 2019 at the Biotechnology Laboratory of the University of Medellín, located in the city of Medellín (6°13'52''N - 75°36'41''W), Antioquia, Colombia.
Microorganisms
Seven yeasts (GRB-LB01, GRB-LB02, GRB-LB05, GRB-LB06, GRB-LB11, GRB-LB12, and GRB-LBL13) isolated by the GRINBIO research group of the University of Medellín from liquid bioferments of MM were used (with a request for access to genetic resources filed No. E1-2021-20477 of June 16, 2021) and selected for their antagonistic potential for the control of Colletotrichum sp. and Fusarium sp. assessed in previous research. The microorganisms were reactivated from cultures preserved on slant agar at 4 °C, using Petri dishes with Yeast Extract Peptone Dextrose agar (YPDA) composed by yeast extract (10 g L-1), peptone (20 g L-1), D-glucose (20 g L-1), bacto-Agar (15 g L-1) at pH 4.5, and incubated at 25 °C for 48 h.
Phosphorus (P) solubilizing capacity in vitro
The evaluation of phosphorus solubilizing capacity under in vitro conditions was evaluated in solid and liquid media.
Evaluation in solid medium: a 0.5 cm diameter disk was transferred with a colony of the selected yeasts (GRB-LB01, GRB-LB02, GRB-LB05, GRB-LB06, GRB-LB11, GRB-LB12, GRB-LBL13) to the National Botanical Research Institute Phosphate growth medium (NBRIP) (Nautiyal, 1999) which contains Glucose (10 g L-1), (NH4)2SO4 (0.15 g L-1), KCl (0.2 g L-1), MgCl2.6H2O (0.25 g L-1), agar (20 g L-1) and Ca3(PO4)2 (5.0 g L-1). The solubilization capacity was determined using the methodology proposed by You et al. (2020) by the appearance of a transparent halo around the colonies after seven days of incubation at room temperature (22 ± 2°C) and the Solubilization Index of Phosphorus (SIP) using Equation 1.
Evaluation in a liquid medium: the solubilization of phosphorus in liquid medium was measured following the molybdenum blue methodology described by Lasso (2007). A calibration curve was previously prepared, in concentrations of 0, 0.2, 0.4, 0.6, 0.8, 1, and 1.2 mg L-1 of KH2PO4, later, 5 mL of each of these standards were taken and 800 µl of the solution composed of 15 mL sulfuric acid (0.245 g mL-1), 1.5 mL potassium antimony tartrate (0.0034 g mL-1), 4.5 mL ammonium molybdate (0.04 g mL-1), and 9 mL ascorbic acid (0.0176 g mL-1) were added. The absorbance was read at 890 nm using a spectrophotometer, and the reaction time was between 10 to 30 minutes.
To determine the amount (mg) of phosphorus solubilized by each yeast, it was inoculated with 1 mL of yeast suspension at a concentration of 1 x 106 cells/mL in 100 mL of liquid culture medium composed of glucose (10 g L-1); NH4Cl (1 g L-1); MgSO4.7H2O (0.4 g L-1); CaCl2.2H2O (0.2 g L-1); KCl (1.87 g L-1) and phosphate rock (5 g L-1). The treatments were kept in the dark and shaking at 100 rpm at room temperature (22 ± 2 °C) for six days. Samplings were carried out on days 0, 1, 3 and 6, then the microbial suspensions were centrifuged at 10,000 rpm for 12 min in a centrifuge and the supernatant was filtered (cellulose nitrate filter 0.2 µm) for the pH measurement (portable multimeter) and the quantification of Soluble Phosphorus (PS), which was carried out following the same procedure carried out with the standards and comparing the absorbance results obtained at 890 nm with the calibration curve. The uninoculated medium was used as a negative control.
The PS produced by the yeast activity was calculated by subtracting the PS concentration of the uninoculated medium (resulting from non-biological activities) from the final PS concentration generated in the inoculated medium. All the glassware used was previously washed with a 5 % dilute HCl solution and rinsed with distilled water to eliminate possible contamination with residual phosphorus from commercial detergents (Lasso, 2007).
In vitro potassium (K) solubilizing ability
The evaluation of potassium solubilizing capacity under in vitro conditions was evaluated in solid and liquid media.
Evaluation in solid medium: the methodology proposed by You et al. (2020) was used with some variations for which the yeasts were seeded with the NBRIP medium consisting of KCl (0.2 g L-1), MgCl2.6H2O (0.25g L-1), (NH4)2SO4 (0.15 g L-1), D-glucose (10 g L-1), Agar (20 g L-1) modified with KNO3 (5 g L-1) and bromocresol purple as pH indicator (0.124 g L-1), which has a violet color at pH equal to or greater than 6.8 and yellow color at pH equal to or less than 5.2, making visible the formation of a yellow halo around the colony as a consequence of the release of organic acids. This medium was inoculated with 0.5 cm diameter discs resulting from the reactivation processes of each of the seven yeasts included in the study. The potassium solubilization capacity was read after seven days of incubation under laboratory conditions at room temperature (22 ± 2 °C), and the Potassium Solubilization Index (SIK) was determined using Equation 1.
Evaluation in liquid medium: This test was carried out in 100 mL of a liquid medium composed of glucose (10 g L-1); NH4Cl (1 g L-1); MgSO4.7H2O (0.4 g L-1); CaCl2.2H2O (0.2 g L-1); NaCl (1 g L-1) and potassium feldspar (5 g L-1) as a source of insoluble K. The liquid media were inoculated individually with 1 mL of yeast suspensions obtained from the reactivation processes of each of the seven yeasts included in the study and adjusted to a concentration of 1 x 106 cells/mL. The cultures were kept at room temperature (22 ± 2 °C) in dark conditions and shaking at 100 rpm for six days. Samplings were carried out on days 0, 1, 3 and 6, for which, the microbial suspensions were centrifuged at 10,000 rpm for 12 min and the supernatant was filtered (Cellulose nitrate filter 0.2 µm) for pH measurement and the quantification of Soluble Potassium (KS) which was performed by atomic absorption spectrophotometry by the company Biofertilizar®. As a control, uninoculated culture media were used. The amount of KS produced by yeast activity was calculated by subtracting the KS concentration of the non-inoculated medium (resulting from non-biological activities) from the final concentration of KS generated in the inoculated media.
Indol acetic acid production (IAA)
The standardization of the tests for the quantification of IAA was carried out using the colorimetric technique based on the use of the Salkowski reagent (12 g L-1 of FeCl3 in H2SO4; 7.9 M) as described by Ullah et al. (2013). AIA solutions were prepared in concentrations of 0, 5, 10, 15, 20 and 25 mg L-1 of AIA, subsequently, 1 mL of each of these standards was taken and 2 mL of Salkowski reagent were added; the reaction was allowed to stand for 30 minutes under dark conditions at room temperature, after this time, absorbances at 530 nm were read on a spectrophotometer.
For the quantification of the IAA production capacity of the seven yeasts, individual liquid cultures of each yeast were made by adding 1 mL of inoculum at a concentration of 1x106 cells/mL in 100 mL of trypticase soy broth. The liquid media were kept shaking at 100 rpm at room temperature (22 ± 2 °C) for 96 hours (Celis Bautista & Gallardo, 2008). For the quantification of IAA, the liquid media were centrifuged for 10 minutes at 5000 rpm, and 1 mL of supernatant was recovered for the quantification of IAA produced following the same procedure carried out with the standards and comparing the absorbance results obtained at 530 nm. With the calibration curve.
Cellulolytic capacity of yeast
The ability of yeasts to produce cellulases was evaluated qualitatively as a vitally important mechanism for the survival of the isolates in the soil. The cellulase production capacity in yeasts was determined using the methodology proposed by Sazci et al. (1986) for which Petri dishes were prepared with CMC medium composed of peptone (2.5 g L-1), carboxymethylcellulose (CMC) (10.0 g L-1), yeast extract (2.5 g L-1), (NH4)2SO4 (0.5 g L-1), CaCl2 (0.5 g L-1), KH2PO4 (0.1 g L-1), K2HPO4 (0.1 g L-1) and agar (15 g L-1). This medium was individually inoculated with 0.5 cm diameter yeast discs obtained from previously reactivated cultures. Subsequently, the petri dishes were incubated at 25 °C for 72 hours. At the end of the incubation period, for the determination of the cellulolytic capacity of the yeasts, the petri dishes were flooded with 0.1 % (w/v) of Congo red reagent and left to rest for 15 min, and finally washed with 1 M NaCl (Rawway et al., 2018). In this qualitative test, the formation of a clear hydrolysis zone around the colonies was used as an indicator of cellulose degradation and the presence of cellulolytic enzymes.
Molecular identification
Molecular identification was carried out for the yeasts with the highest agronomic potential due to their solubilizing activity of P and K and their production of IAA. The identification was carried out by the Colegio Mayor de Antioquia University Institution, where DNA extraction, quantification, and purity evaluation were carried out in NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE), and the amplification of the ITS region by polymerase chain reaction (PCR) with the ITS1 (5'-TCCGTAGGTGAACCTGCGG-3') and ITS4 (5'-TCCTCCGCTTATTGATATGC-3') primers recommended for fungi. The obtained and purified PCR products were sequenced by Macro Gen Inc. To determine the possible identity of the isolates, the sequences of each primer were purified and aligned using the Geneious® program version 9.1.5 and compared in the GenBank database of the «National Center for Biotechnology Information» using BLASTN (Madden et al., 1996).
Statistical analysis
A completely randomized design (DCA) was used, where the factor was the microorganism in seven levels and three replications, to evaluate the effects on the solubilization index of potassium (SIP) and phosphorus (SIK), the variation of pH, the concentration (mg L-1) of soluble P and K, and the IAA concentration (mg L-1). In all cases, the variables were expressed as the ± the standard deviation. To determine the effects of the treatments on the mentioned response variables, a one-way ANOVA was implemented for an analysis of variance and a Tukey test (α = 0.05) for multiple comparisons of means, using the IBM SPSS 25 statistical software (IBM Corporation, 2017). Previously, normality was confirmed with the Shapiro Wilk test and independence of variances with the Levene test. A non-parametric test (Kruskal-Wallis) was applied for the analysis of the phosphorus solubilization index and a comparison between pairs to determine the significant differences (α=0.05).
Results
Phosphorus (P) solubilizing capacity
The phosphorus solubilization activity of yeasts evaluated under in vitro conditions showed differences between solid and liquid media. In vitro evaluation in solid medium after seven days of incubation in NBRIP agar, only GRB-LB05 presented solubilization halo with a mean value of Phosphorous Solubilization Index (SIP) of 1.06 ± 0.01 (Table 1). The other yeasts did not register solubilization halo under the study conditions.
Table 1 Solubilization index of phosphorus (SIP) and potassium (SIK) for yeasts after seven days of culture and identity of the yeasts. Biotechnology Laboratory of the University of of Medellín, Medellín - Colombia, 2019.
| Isolated | Solubilization index* | Molecular identification | |||
| Potassium (K) | Phosphorus (P) | Microorganism | Access number | Percentage of similarity | |
| GRB-LB01 | 10.37 ± 0.51 a | 0 ± 0 b | unidentified | unidentified | unidentified |
| GRB-LB02 | 6.39 ± 0.14 c | 0 ± 0 b | Pichia kudriavzevii | KJ706301.1 | 94 |
| GRB-LB05 | 9.04 ± 0.33 b | 1.06 ± 0.01 a | Kluyveromyces siamensis | MW617895.1 | 98 |
| GRB-LB06 | 9.18 ± 0.38 b | 0 ± 0 b | Candida haemulonis | MG637448.1 | 99 |
| GRB-LB11 | 6.45 ± 0.15 c | 0 ± 0 b | Pichia kudriavzevii | KP674518.1 | 99 |
| GRB-LB12 | 6.37 ± 0.19 c | 0 ± 0 b | Suhomyces xylopsoci | MZ269243.1 | 97 |
| GRB-LB13 | 9.29 ± 0.46 b | 0 ± 0 b | Candida haemulonis | MN172343.1 | 84 |
In vitro evaluation in liquid medium showed that when the changes in pH in liquid media enriched with phosphate rock as a source of P and inoculated with yeasts (Figure 1) were analyzed, a decrease in pH was observed towards day one of culture. However, the lowest values were reached on day three, being the yeasts GRB-LB01 and GRB-LB06 (Figure 1A) which presented the highest pH values low, which differ statistically among themselves and with the other treatments including the control.
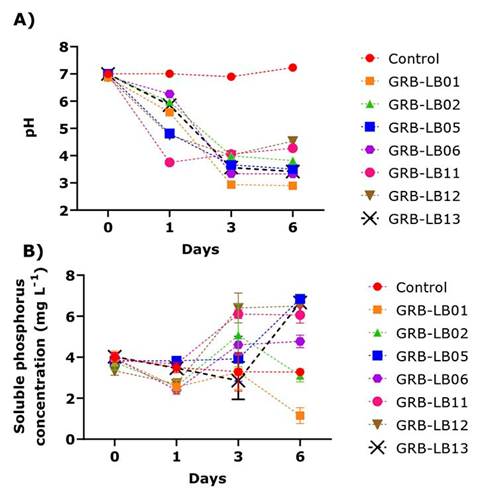
Figure 1 Kinetics of variables evaluated for the solubilization of phosphoric rock as the sole source of phosphorus in liquid medium by the isolates during six days of incubation. A) pH variation. B) Variation of soluble phosphorus concentration (mg L-1). Biotechnology Laboratory of the University of Medellín, Medellín- Colombia, 2019.
The PS quantification tests (Figure 1B) showed that, on the first day of incubation, GRB-LB11, GRB-LB05, and GRB-LB13 presented statistically similar values to the control, the other yeasts, on the first day of culture, had a reduction in PS, with values between 2.40 to 2.69, lower than those observed at the beginning of the culture (3.83 to 4 mg L-1). For day 3, the PS obtained in the cultures of the yeast GRB-LB05 remained statistically constant and the yeasts GRB-LB12 and GRB-LB11 presented statistically higher values than the control, increasing the PS by 2.48 and 2.18 mg L-1 respectively.
The analysis carried out on day 6, allowed evidence of increases and decreases of the PS in the different yeast cultures. GRB-LB05, for example, reached the highest PS in this study with an increase of 3.57 mg L-1 compared to the control, this yeast also stood out in solid medium with a SIP of 1.06 ± 0.01. For this day, the yeasts GRB-LB13, GRB-LB12, GRB-LB11 and GRB-LB06, PS concentrations also increased as to the control, although to a lesser extent, with increases of 3.4, 3.23, 2.78 and 1.46 mg L-1, respectively.
Potassium (K) solubilizing capacity
The response of the yeasts to potassium solubilization depended on the conditions in which they were cultured, showed differences between the in vitro technique in solid medium in relation to the liquid medium.
In vitro evaluation in solid medium showed that after seven days of culture on modified NBRIP agar, all yeasts showed a turn of the solid medium from purple to yellow. The quantifications of the diameters of the halos around the yeast colonies revealed differences between the SIKs. In the tests, the yeasts with the highest SIK were GRB-LB01, GRB-LB013, GRB-LB06 and GRB-LB05 (Table 1), however, the statistical analyzes allowed the identification of three homogeneous groups, separating the yeast GRB-LB01 from the other three with the highest SIK, considering it as the one with the best result.
In vitro evaluation in liquid medium evidenced that when analyzing the pH kinetics, it was generally observed that the highest rate of decrease in pH occurred in the first 24 hours of culture for all yeasts, being GRB-LB11 and GRB-LB12 which generated the highest acidifications for this day (Figure 2A). The lowest pH values reached in this study were observed between days 3-6 of culture, being the GRB-LB06 yeasts GRB-LB13 and GRB-LB01 which generated the highest acidifications.
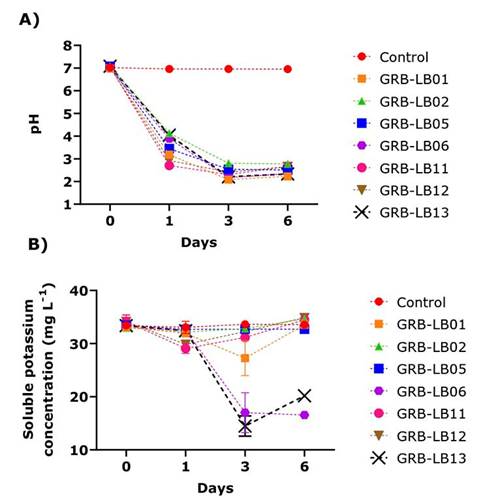
Figure 2 Kinetics of variables evaluated for the solubilization of feldspar as the only source of potassium in liquid medium by the isolates during six days of incubation. A) pH variation. B) Variation of soluble potassium concentration (mg L-1). Biotechnology Laboratory of the University of Medellín, Medellín- Colombia, 2019.
KS kinetics showed a significant reduction on day one for the GRB-LB12 and GRB-LB11 yeasts, for the others, the values of KS were similar to the control. For the third day, no yeast presented solubilization superior to the control and the values of GRB-LB12 and GRB-LB11 increased, reaching the KS of the control, additionally, the quantification of KS on day three showed consumption of 42 % for GRB-LB06 and 50 % for GRB -LB13. On day six, differences were observed in the KS values of the yeast cultures with respect to the control, the highest values were found in the GRB-LB12 cultures and GRB-LB02, which, when compared with the control, indicated a K solubilization of 1.32 and 1.12 respectively.
Indol acetic acid production (IAA)
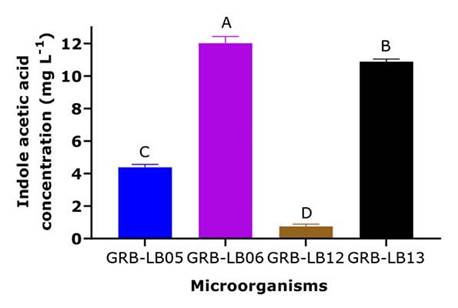
The results of the colorimetric tests performed with the Salkowski reagent indicated that only the yeasts GRB-LB05, GRB-LB06, GRB-LB12 and GRB-LB13 produced IAA (Figure 3).
Cellulolytic capacity
The colorimetric test with Congo red, carried out after 72 hours of incubation at room temperature, showed that all the yeasts had a cellulose degradation halo with a diameter between 1.1- 1.5 cm, which indicated the capacity to produce cellulases.
Molecular identification
The amplification of the ITS-PCR of the DNA extractions of the isolates under study allowed the identification of yeasts with biofertilizing and / or growth-promoting activity (Table 1) allowed to identify the presence of the yeasts Pichia kudriavzevii, Kluyveromyces siamensis, Candida haemulonis, and Suhomyces xylopsoci. GRB-LB01 yeast was not identified, since it did not show biofertilizing and / or growth-promoting activity of interest in liquid medium.
Discussion
The solubilization index of potassium (SIP) values obtained in this study are lower than those reported by Pandi et al. (2019) for rhizospheric yeasts (SIP between 28.89 - 56.67), indicating no success in the initial tests performed on solid media. However, when comparing the solubilization results obtained on day six in liquid media enriched with phosphate rock, it can be seen that the solubilization values of the yeasts GRB-LB05, GRB-LB13, GRB-LB12, GRB-LB11, and GRB-LB06 (1.46 - 3.57 mg L-1), are similar to those reported by Boubekri et al. (2021) for Streptomyces in liquid cultures enriched with phosphate rock (SIP between 0.1 and 32 mg L-1). Other studies such as those of Borges de Oliveira et al. (2019) and Jeberlin Prabina et al. (2019) reached a solubilization rate of tricalcium phosphorus of 150 mg L-1 at 6 days of culture with Torulaspora globosa and 386 mg L-1 with the Issatchenkia terrestrial at 3 days of culture, values that are much higher than those obtained with the studied yeasts.
When comparing the results of the P solubilization tests carried out in a solid medium NBRIP and in a liquid medium for the selection of solubilizing microorganisms, substantial differences were found in the sensitivity obtained, since, in a solid medium, only the yeast GRB- LB05 presented a solubilization halo (SIP: 1.06 ± 0.01), while, in the liquid cultures, in addition to GRB-LB05, strains GRB-LB13, GRB-LB12 and GRB-LB11 also registered PS on day 6 (Figure 1). These results suggest that the production of halos of solubilization in a solid NBRIP medium should not be considered as the only test for the selection of P-solubilizing microorganisms and highlights the need for a confirmatory test in a liquid medium. This type of recommendation has already been suggested by other authors such as Bashan et al. (2013) who report variability in the results of P solubilization capacity when cultivating microorganisms in solid and liquid selective media and suggest that false negatives or false positives could be generated.
The K solubilization response in solid media reported by other authors, qualifies the results of this investigation to be considered promising, since the SIK values obtained in Petri dish for GRB-LB01 yeasts , GRB-LB013, GRB-LB06 and GRB-LB05 (Figure 2) are similar to those reported by Pandi et al. (2019) and Setiawati and Mutmainnah (2016) who indicated successful results when obtaining a SIK value of 9.53 cm in the tests carried out with the yeast Asb4 and of 10.99 cm when working with the yeast encoded as SY5, respectively. When analyzing the solubilization kinetics of K in the liquid medium supplemented with feldspar, the most promising SK results were observed with GRB-LB12 and GRB-LB02 (Figure 2), however, these values are lower than those reported by Boubekri et al. (2021) who obtained a range of 3 to 18 mg L-1 of KS with Streptomyces isolates and those reported by Mohamed et al. (2017) who obtained SK values of 8.11 to 13.21 mg L-1 of KS after 20 days of incubation when cultivating strains of Pichia anomala and Rhodotorula glutinis.
When comparing the results of SK obtained in solid media and with those obtained in liquid media, it was expected that the yeasts GRB-LB05, GRB-LB13, and GRB-LB06 would stand out in liquid medium as solubilizers, however, no KS was found for these yeasts on no day of study, finding, on the contrary, a K consumption demonstrated with KS records lower than those of the control. These results highlight the possibility of obtaining false positives in KS-selective solid media, demonstrating the unreliability of the Petri dish tests for the selection of solubilizing yeasts. These differences between solid and liquid media make it difficult to select promising SK strains, it has been reported before (Rajawat et al., 2016), where, as a result of the difficulty to standardize a promising medium for the rapid identification of KS microorganisms, a different medium than the one used in this study was required.
In both the P and K solubilization tests in the liquid medium, a decrease in pH was observed, however, the yeasts with the lowest pH did not show a direct relationship with the increase in PS and KS values, this results do not agree with what was reported by Moreno Quevedo et al. (2015) where cultures inoculated with Aspergillus niger indicated the reduction in pH as an indicator of the solubilization capacity of P, in their studies the values of pH 3.58 were those who obtained a higher concentration of soluble phosphorus (42.3 mg L-1); and Bagyalakshmi et al. (2017) achieved the highest solubilization of K (41.91 mg L-1) with Pseudomonas nitroreducens when reducing the pH to values of 4.5 being the lowest of all their study. According to reports found, the acidification of the medium occurs as a result of the synthesis of organic acids such as oxalic, formic, citric, malic, propionic, tartaric acid, among others (Kumar et al., 2016; Xiao et al., 2013), this reduction in pH induces the ability to release K and P from minerals to bring them to their available solution form (Teotia et al., 2016). However, the solubilization of P and K can be carried out through other mechanisms of action that could help to explain why in this study the solubilization did not show a direct relationship with the reduction in pH; Mechanisms have been found that involve the production of phytase and phosphatase enzymes (Din et al., 2019), slime production or acidic exo-polysaccharides (Prabhu et al., 2019; Sattar et al., 2019), chemical weathering based on of carbonic acid (Sattar et al., 2019) and chelation (Karmakar et al., 2018).
The solubilization rate depends on environmental factors that include the nature of the minerals, the source of carbon and nitrogen availability in the medium as well as the general growth conditions, it also depends on the characteristics of microorganisms such as the quantity and quality of acids produced by it (Hernández-Leal et al., 2011; Teotia et al., 2016). In this study, soluble levels of P (PS: 3.84 mg L-1), and K (KS: 33.47 mg L-1) were found at the beginning of the tests before the inoculations, possibly as a result of the sterilization of the minerals and of the use of partially acidulated phosphate rock (pH 6.30) as indicated by Samanego Vivanco (2018). The presence of soluble P, and K in the culture media in the initial phase may help to explain the reduction of the response of the solubilization of feldspar in the cultures of GRB-LB01, and GRB-LB02, and phosphate rock in the cultures of the GRB-LB01, GRB-LB05, GRB-LB06 and GRB-LB13 yeasts. It is possible that not having the stress generated by the macronutrient deficit, it did not activate the microbial mechanisms that allow the solubilization of the minerals present in the rocks, reducing their activity to the consumption of the available P and K. In the studies carried out by Osorno Bedoya and Osorio Vega (2017) was evidenced that a higher concentration of soluble P at the beginning of the process decreased the solubilization of phosphate rock by Mortierella sp. while in results reported by Flatian et al. (2021), despite presenting initial KS, found solubilization of feldspar by unidentified bacteria and fungi.
The yeasts GRB-LB05, GRB-LB13, GRB-LB06 and GRB-LB12, also showed the ability to synthesize IAA (Figure 3); this phytohormone is known as a plant growth promoter since it can regulate and improve plant growth, being an important factor in determining the agricultural potential of a microorganism. When comparing the IAA, levels reached by the yeasts understudy with the values reported by other authors such as Nakayan et al. (2013) and Nutaratat et al. (2014), the potential of some of the yeasts can be evidenced, they reported promising IAA production values in a range between 8.9 - 10.6 mg L-1 for Rhodotorula mucilaginosa cultures in YPD media, values that were exceeded by the GRB-LB06 and GRB-LB13 yeasts. Additionally, when evaluating the classification proposed by Khalid et al. (2004), the yeasts GRB-LB05, GRB-LB13, and GRB-LB06 can be classified as high producers of IAA and the yeast GRB-LB12 as low producer (Figure 3).
When recommending an IAA-producing microorganism it is also important to consider that IAA stimulates plant growth only within a concentration range. Peña-Yam et al. (2016) reported an IAA production between 4.0 to 24.3 mg L-1 indicating that these are sufficient concentrations to promote the growth of Capsicum annuum L. Hernández-Hernández et al. (2018) generalize the range AIA indicating that this should be between 5 to 72 mg L-1 being ineffective when it is very low and toxic when it is very high. These results again support the agronomic potential of the GRB-LB06 and GRB-LB13 yeasts.
Although the results show the potential of these yeasts, they could be optimized following the recommendations of Rojas Contreras et al. (2010), by adding tryptophan to trypticase soy broth, which only provides low concentrations of this precursor (100 mg L-1). Some studies that show this potential improvement was presented by Liu et al. (2016) who managed to improve IAA production by working with Saccharomyces sp. and Torulaspora globosa in YPD media with tryptophan (0.1 % w / v), finding an increase in IAA concentration from 13 mg L-1 to 287 mg L-1; and Thais et al. (2019) that studied the synthesis of IAA with Torulaspora globosa in supplemented PD media, obtaining a maximum concentration of 669 mg L-1, values that would have to be carefully analyzed to avoid reaching toxic levels of IAA.
The cellulase production found for all yeasts in this study is an indicator of their ability to make use of plant organic matter as a carbon source, this being a factor that adds to their biofertilizer potential. Numerous species of microorganisms of biofertilizer interest have been reported with cellulase synthesis such as Penicillium, Aspergillus, Bacillus, Azotobacter, and Saccharomyces (Marwanto Bustaman et al., 2021), these studies indicate the advantages that these enzymes provide to microorganisms to survive in the soil for longer periods. Additionally, studies such as A'Bear et al. (2014), Asoegwu et al. (2020) and Fikrinda et al. (2020), indicate that cellulase-producing microorganisms can stimulate plant growth directly and indirectly, through the decomposition of soil organic matter, the regulation of the availability of nutrients such as phosphorus and potassium and the generation of phytohormones.
According to the identification results, the yeasts were taxonomically classified into 4 species (Table 1), of which only for Pichia kudriavzevii (GRB-LB02, GRB-LB11) there were reports in the literature that highlight their agricultural potential due to their enzyme synthesis and its potential in promoting plant growth. Boontham et al. (2019) point out its capacity for extracellular phytase production and IAA production was recorded by Limtong et al. (2014). Unlike what was reported by other authors, in the present study, neither of the two yeasts identified as Pichia kudriavzevii (GRB-LB02, GRB-LB11) stood out for their ability to solubilize phosphorus and produce IAA, however, they did show potassium solubilization ability, this report being one of the first to mention such activity for P. kudriavzevii.
For the other isolates identified, little information was recorded and no reports of agricultural interest were found, except for the one presented in this study, in which the solubilizing capacity of P and synthesis of IAA by Kluyveromyces siamensis (GRB- LB05), increasing the information on this microorganism for which only reports of its killer capacity against pathogenic Metschnikowia bicuspidata were found in crab from an isolation in mangroves in Thailand (Buzdar et al., 2011; Kunthiphun et al., 2019). The GRB-LB12 isolate identified as Suhomyces xylopsoci, previously known as Candida xylopsoci (Kurtzman et al., 2016) is one of the most promising of the study, however, it has not been studied much yet (Jacques & Casaregola, 2019). Its presence has been reported in silage processes with a high production of organic acids, which, as mentioned above, are closely associated with the solubilization of phosphorus and potassium and could be an important factor that would explain the results achieved with this strain. GRB-LB06 and GRB-LB13 identified as C. haemulonis, although they presented good capacity for the production of IAA and solubilization of P, it has been reported in the literature as a causal agent of infections and nosocomial diseases (Sipiczki & Tap, 2016) so if in the future it is desired to use these as efficient microorganisms, it would be necessary to carry out pathogenicity tests, ensuring innocuousness and safety for animals, humans and the environment.
The analysis of the capacities studied for the yeasts, previously selected for their biofungicidal potential, indicates that the antagonist yeasts C. haemulonis (GRB-LB13) and S. xylopsoci (GRB-LB12) are those with the highest agronomic potential due to the wide range of responses found regarding the solubilization of PK in the liquid medium, the production of IAA and CMC that show their biofertilizer and growth-promoting action that allows their inclusion in the category of efficient microorganisms which could act very well in the consortium to promote the sustainable development of agriculture. Numerous articles seek to isolate and identify microorganisms with more than one biological activity of interest, as in the case of Nath et al. (2017) that document bacteria that solubilize K and P from different minerals; some studies highlight the biostimulant and antagonist advantages of yeast (Hernández-Fernández et al., 2021).
Conclusions
It is concluded that the antagonistic yeasts isolated from liquid MM bioferments have a biofertilizing and growth-promoting capacity, with GRB-LB12 (Suhomyces xylopsoci) being the one that stands out in the solubilization of P, K, IAA production and cellulolytic capacity that allows it to survive in the soil, finally higher mineralization of organic residues is expected with yeast.