Introduction
Natural hybridization is a relatively common phenomenon, which has played an important role in plant evolution. Hybridization has been considered a great force in the speciation and diversity of angiosperm species (Grant 1981, Soltis & Soltis 2009). Within the Orchidaceae, the existence of natural hybrids is well known. They are capable of developing viable seeds through intraand intergeneric crosses; this fact probably allows some gene exchange in nature to be added to the gene pool of the species (Pupulin 2007, Soltis & Soltis 2009). However, species also maintain reproductive isolation through different prezygotic mechanisms, such as phenology, fragrances, pollinators and geographic distribution (Pupulin 2007, Faegri & Van Der Pijl 2013).
About 10% of Neotropical orchids are pollinated exclusively by orchid bees (Apidae: Euglossini; Ramírez 2019), whose males visit flowers and other sources in search of aromatic substances, which are used to attract females (Eltz et al. 1999). The reproductive isolation barriers used by these groups of orchids, in addition to those already mentioned, can be mechanical in function. For example, the area of the bee’s body where each orchid species positions its pollinia is different, as is the shape of the pollinaria, and how it interacts with the stigma of the flower, through the size, form and behavior of the bee (Dressler 1968). Furthermore, these groups of orchids also employ chemical reproductive barriers; that is, the fragrance used by orchid species of the same genus usually have different compositions when they are sympatric (Dressler 1968, van der Pijl & Dodson 1966).
The subtribe Stanhopeinae are one of these groups of orchids pollinated exclusively by euglossine bees (Dressler 1968, 1993, Williams & Whitten 1983); with Stanhopea Frost ex Hook and Gongora Ruiz & Pavón being the largest genera. Although they are prone to hybridize, natural hybrids in the Stanhopeinae are very rare in nature (Gerlach 2003), and the few that have been found so far belong to Stanhopea (Dressler 1968, 1993, Jenny 1993a). Gongora comprises around 60-70 species, distributed from southern Mexico to South America, along the slopes of the Andes, as well as in regions of Venezuela, the Guianas, and Brazil (Hetherington-Rauth & Ramírez 2016, Jenny 1993b). Taxonomically, the genus is subdivided into three subgenera based on lip morphology: Portentosa Jenny, Acropera (Pfitzer) Jenny (which is subdivided into two sections: Acropera and Armeniaca), and Gongora (Pfitzer) Jenny (which is subdivided into five sections: Aceras, Gratulabunda, Gongora, Grossa, and Truncata) (Chase et al 2009). Jenny (1993b) considers a third section within the subgen. Acropera (sect. Cassidea), however, in this study, the most recent classification is used.
The objective of this work is to report a hybrid specimen collected in nature in the department of Copán, Honduras, found by chance during fieldwork carried out by some of the authors (HV, AA, EM). The discovered plant presents intermediate characters between species of Gongora belonging to two different subgenera, and we hypothesize that the probable parental species are G. truncata Lindl. and G. batemanii (Lindl. ex Rchb.f.) Henshall ex Mabb. & Jenny, both of which are found in the area of discovery. We also seek to clarify the identity of another specimen that likely corresponds to the same hybrid described here, and comment on some relevant aspects of the ecology and pollination mechanisms of the parent species.
Materials and methods
Study area.- The discovery site is located north of the department of Copán, Honduras, a few kilometers from the archaeological reserve of the same name, at an elevation of 1650 m. The climate is classified as tropical rainy, with an average annual temperature of 26°C and an average annual rainfall of 1337 mm. Although, the vegetation is from the tropical humid forest, some parts of the discovery area are considered ecotones with pine forests, with large trees and abundant epiphytes, among which are: Coelia densiflora Rolfe, Epidendrum cardiophorum Schltr., E. isthmi Schltr., E. laucheanum Rolfe, E. repens Cogn., Gongora truncata, G. batemanii, Maxillaria densa Lindl., Pleurothallis pansamalae Schltr., Prosthechea vitellina (Lindl.) W.E.Higgins, Scaphyglottis prolifera (Sw.) Cogn., and S. fasciculata Hook. Among the most common tree species are: Clethra occidentalis (L.) Kuntze, Liquidambar styraciflua L., Myrsine coriacea (Sw.) R.Br., Pinus maximinoi H.E.Moore, and Vochysia guatemalensis Donn.Sm. The exact location of the plant is not revealed in the present paper to avoid illegal collection to which many orchids are subjected. However, exact details on the collecting locality can be found on the labels of the preserved specimen deposited at EAP.
Results
Description of putative parental species.- Gongora truncata Lindl. belongs to the section Truncata of the subgenus Gongora and is distributed throughout Mexico (Veracruz, Oaxaca and Chiapas), Belize, Guatemala and Honduras. It is recognized for its showy flowers, reflexed lateral sepals that appear rectangular in natural position, and decurrent, small petals. The white or yellow lip, welded to the foot of the column, is cymbiform and laterally compressed, fleshy and waxy in appearance, concave, complex, hypochile unguiculate, with a very small transverse callus, with an inconspicuous lobe near the base on each side, and with a sharp rib ending in a retrorse bristle on each side. The epichile is oblong-ovate with a recurved apex (Salazar in Hágsater & Salazar 1990). Phenology: January to June (Beutelspacher 2014) (Fig. 1A). Gongora batemanii (Lindl. ex Rchb.f.) Henshall ex Mabb. & Jenny (synonym: Gongora cassidea Rchb.f.) belongs to the section Acropera of the sub genus Acropera and it is distributed in Mexico (Chiapas), Guatemala, Honduras, and Nicaragua (Ames & Correll 1985). It can be recognized by the arcuate pedicellate ovaries, the broadly elliptic and cucullate dorsal sepal, the oblong to suborbicular-elliptic, obtuse and oblique lateral sepals inserted perpendicularly to the sides of the column. The lip is complex, articulated to the column foot, arched with the callus at the apex; lower portion of lip saccate, thickened and compressed in front of the sac, with a pair of erect parallel lobes arising from the margins of the sac (Ames & Correll 1953). Phenology: February to July (Damon et al. 2012). (Fig. 1B-C). Gongora × copanensis Jiménez Axr, Vega, Alvarado & Mó., nothosp. nov. (Fig. 1D, 2, 3).
TYPE: Honduras. Santa Rita: Copán, tropical rain forest, May 15, 2019. UTM: 287236.24 m E, 1636948.11 m N, 1650 m above sea level, A. Alvarado #001 (holotype, EAP).
Diagnosis: Epiphytic plant, pendulous inflorescence, complex flowers. Intermediate between G. truncata and G. batemanii. It differs from G. truncata by a large callus, the spatulate epichile, cucullate hard dorsal sepal, and the lateral sepals perpendicular to the column. It also differs from G. batemanii by the slightly arched flower pedicel, the absence of the lip articulation, and setaceous projections on the lip.
Plant herbaceous, epiphytic, bifoliate. Pseudobulbs conical, elongate, sulcate. Leaves apical, elliptic, acuminate, three-veined, attached to the pseudobulb by a pedicel. Inflorescence a pendulous raceme, 19 flowers. Ovary pedicelate, incurved, terete, colored dark violet. Flowers 2.7 × 4.6 cm long, showy, not resupinate, fragrant, basically brown, with small purple dots, yolk-yellow lip with violet tips. Dorsal sepal 1.9 cm long, fused to the base of the column, strongly cucullate, ovate-acuminate with slightly retrorse tip. Lateral sepals 1.4 cm long, rolled, perpendicular to the column, obovate. Petals very small, covered by the dorsal sepal, welded to the column, lanceolate, acuminate. Lip fleshy, waxy, complex, semitruncated, divided into hypochile, mesochile and epichile, laterally compressed, apex of epichile and apex of hypochile lobes purple. Hypochile 1 cm long, fused to the foot of the column, fleshy in texture, widening towards the base, grooved towards the apex, yellow, in lateral view saccate on the upper margin, rectangular on the lower, the laminae of the cavity unguiculate, slightly reflexed, with an inconspicuous lobe near the base of the epichile, the lamina conduplicate in its middle part, with two lobes on each side, the anterior large, strongly acuminate with a violet bristles termination, and the posterior small, completely reflexed, ovate-acuminate. Mesochile in lateral view very narrow, with a subquadrate, carinate, concave callus. Epichile 1.1 cm long, spatulate, longitudinally concave, cymbiform, trilobed, median lobe recurved, acuminate, the lateral ones oblong, the epichile separated from the mesochile by an angle of about 15° and a deep sinus. Column 1.5 cm long, covered by the dorsal sepal, semiclaviform, trigonous, arched, winged near the apex. Capsule not seen.
Phenology: flowering observed in May.
Etymology: the name refers to the area where it was discovered, in the department of Copán, Honduras. Copán was an astronomical center of the ancient great Mayan civilization.
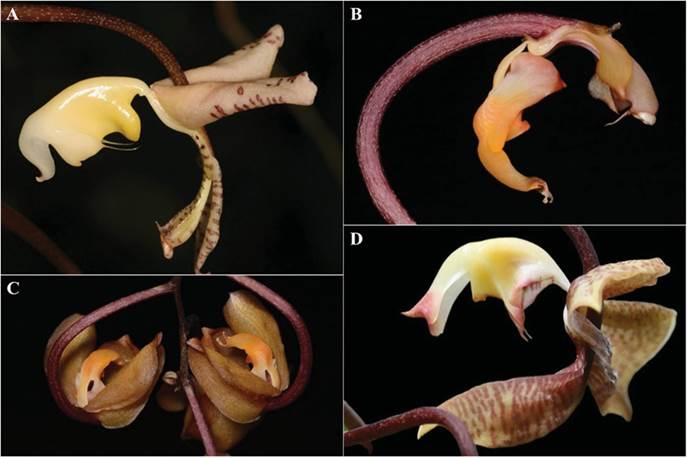
Photographs by Rudolf Jenny (A), José Monzón Sierra (B-C), and Alexander Alvarado (D).
Figure 1 A. Gongora truncata. B. G. batemanii, sepals removed. C. G. batemanii with the sepals in place. D. Detail of the flower of Gongora × copanensis.
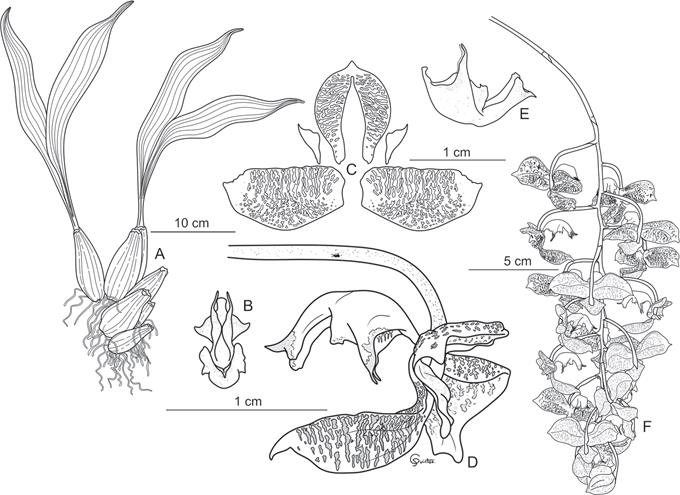
A. Habit and size of the plant.
B. Front view of the lip and view of the inconspicuous basal lobes in profile view.
C. Petals and sepals.
D. Flower, profile view.
E. Profile view of the dissected lip.
F. Inflorescence.
Illustration by Gerardo Quintos Andrade, based on A. Alvarado #001 (EAP).
Figure 3 Illustration of Gongora × copanensis.
Discussion
Gongora × copanensis presents a mixture of exclusive characteristics of members of subgen. Acropera sect. Acropera, and others of members of subgen. Gongora sect. Truncata. The only representatives of these groups in the discovery area where the putative hybrid was found are G. truncata and G. batemanii, which also correspond morphologically to the specimen described here, and whose flowering periods also overlap, for which it is considered as a hybrid between these species. The hybrid shares with G. truncata the shape of the pseudobulbs, the ovary not strongly arched (which positions the lip above the column horizontally and not vertically as in members of the sect. Acropera), and how the hypochile laminae fold, which is similar to G. truncata. Above all, it presents a pair of bristles on the anterior lobes of the hypochile, similar to the typical bristles of the subgen. Gongora, which are absent in all the species of subgen. Acropera. In addition, it shares with G. batemanii the cucullate dorsal sepal and the lateral sepals arranged perpendicular to the column, the callus and the first third of the hypochile exposed by the hypochile lobes, and the spatulate epichile; however, it lacks the joint that allows movement of the lip. Although numerous artificial hybrids have already been recorded for this genus, this is the first record of a natural hybrid for this popular but taxonomically difficult group of orchids.
A specimen with the same floral characteristics as the nothospecies described here, appeared without locality on the digital platform of the Swiss Orchid Foundation at the Herbarium Jany Renz, Botanical Institute, University of Basel, in Switzerland. It was registered under number 2065154 (Fig. 4A-B), and erroneously identified as Gongora saccata Rchb.f. (Fig. 5C). The latter is a synonym of G. seideliana Rchb.f. (Fig. 5A-B), since Reichenbach named the same plant twice (R. Jenny pers. comm. 2020), G. seideliana is a rare species from southern Mexico (García-Matínez & Jiménez-Machorro 2016). The photos and the identification of the specimen on the said platform, which have now been removed from the webpage, were erroneously attributed to Rudolf Jenny, but they do not belong to or were determined by him (R. Jenny pers. comm. 2020). This second plant has the basal lobes of the hypochile that are not markedly retrorse, so they are conspicuous in lateral view, unlike the specimen from Honduras, which has the same lobes retrorse, which makes them inconspicuous in lateral view. However, its shape basically corresponds tho the taxon described here. A third cultivated specimen is also known from a private collection in the same locality of Copán in Honduras, but we had no opportunity to voucher it. The presence of these plants, product of the crossing of the same parents, allows us to infer that sometimes the reproductive barriers between these species are broken, allowing with relative frequency the exchange of genetic material.

Photographs by SORA.
Figure 4 A-B. Specimen 2065154 from the Swiss Orchid Foundation at the Herbarium Jany Renz, Botanical Institute, University of Basel, Switzerland (SORA).
The species of euglossine bees pollinating G. truncata and G. batemanii have not been recorded. This prevents the possibility to glimpsing the probable cause of the breakdown of the reproductive barriers between both species, or to be able to infer the zones where this hybrid could appear based on the distribution overlapping of the involved plant spescies and their pollinators. However, some relevant aspects of the mechanical and chemical barriers of both parent species can be discussed.
Gongora truncata and G. batemanii have different pollination mechanisms. Hetherington-Rauth and Ramírez (2015) describes the hinge mechanism for the Acropera and Cassidea sections of the subgen. Acropera. In this mechanism, the species are pollinated by bees of the genus Euglossa Latreille, and position the pollinarium on the scutellum, with the help of a joint that allows the lip movement of the flowers. For subgen. Gongora sect. Truncata, and based on the observation of South American species, the same authors describe the header mechanism (Fig. 5E), in which the orchids of this group use large bees of the genus Eulaema Lepeletier, that receive the pollinarium behind head. This appears to hold true for the South American members of section Truncata, but not for the Mesoamerican species of this section, which have been observed using the genus Euglossa near their natural ranges (G. truncata: Winter pers. obs. 2022 (Fig. 5D), A. Jiménez pers. obs. 2022; G. seideliana: A. Mendez pers. obs. 2022). These are small bees to which the flowers adhere the pollinia to the scutellum, employing the slide mechanism (typical for the rest of the subgenus they belong to), in which the bees slip from the lip towards the column of the flower. Therefore, despite having different pollination mechanisms, both parent species place their pollinia on the same part of the bees’ body.
As for the chemical barriers, the exact composition of the fragrance cocktail of the parent species is unknown. However, Hetherington-Rauth and Ramírez (2016) found that the terpene a-farnesene is part of the fragrance cocktail of both. Some authors have considered this terpene unattractive for Euglossini bees (Ackerman 1989, Williams & Dodson 1972, Williams & Whitten 1983). Nevertheless, the authors also found that this and other terpenes considered unattractive, appear in large quantities in some species of Gongora and related genera, representing up to 75% of the total fragrance. They comment that this percentage suggests a greater significance for these types of compounds in the function of the perfume due to the high cost that the synthesis of terpenes represents for the plant kingdom (Gershenzon 1994). Furthermore, substances considered unattractive are known to interact with attractive ones, with this chemical interaction having repercussions on bee behavior (Hetherington-Rauth & Ramírez 2016, Williams & Dodson 1972). How attractive and unattractive chemicals interact with bees, is a topic that needs much more research. This opens the possibility of a better understanding of natural hybridization in orchids.
Sharing one or more pollinator species is common in the Stanhopeinae (Hetherington-Rauth & Ramírez 2015, Roubik & Hanson 2004, van der Pijl & Dodson 1966). The barriers used by euglossine-pollinated orchids must be effective because natural hybrids are infrequent, but hybridization occurs when these barriers fall for some reason (Dressler 1968).

Photographs by Eduardo Pérez García (A), Rudolf Jenny (B-C), Diana Winter (D), and José Mesa Londoño (E).
Figure 5 A. Gongora seideliana. B. Illustration of Reichenbach’s G. seideliana. C. Illustration of G. saccata from Reichenbach, Herbarium of Natural History, Vienna (W). D. Gongora truncata and Euglossa sp. E. Gongora tracyana and Eulaema bombiformis.
Natural phenomena such as natural hybridization and its genetic consequences, are receiving enthusiastic attention from researchers (Soltis & Soltis 2009). Therefore, reporting phenomena like this is of great importance to understanding the way and frequency in which species interact and incorporate genetic characteristics among themselves. It is known that some natural hybrids sometimes manage to establish healthy populations, developing a new evolutionary route and, therefore, speciation (Dressler 1968, Soltis & Soltis 2009). Observing these changes help to understand the complexity of these interactions and the extraordinary plasticity of the chemical and evolutionary ecology in the subtribe Stanhopeinae and the tribe Euglossini. Furthermore, the role of ecotones and problems, such as deforestation and ecosystem fragmentation, could affect the frequency of these phenomena.












 uBio
uBio 



