Introduction
Sri Lanka, with a total land area of 65,610 km2 is a tropical island located in the Indian Ocean bearing a rich and unique biodiversity. The Central Highlands complex is situated in the south-central part of the island and is considered as a super biodiversity hotspot (UNESCO 2010). More than 50% of the endemic vertebrates, 50% of the endemic flowering plants and more than 34% of the endemic trees, shrubs, and herbs are reported from these diverse montane rainforests and associated grassland areas (Gunawardene et al. 2007, Ministry of Environment 2012). Orchidaceae is among the largest families of flowering plants in the country with 189 known species, belonging to 78 genera, including 55 endemic species (Fernando 2013). More than any other plant family, Orchidaceae has a high proportion of threatened genera with most containing threatened species (Swarts & Dixon 2009). Considering the Sri Lankan orchids, 70.6% of the species including 84% of the endemics are categorized as threatened. Within this 8.6% of the species are Critically Endangered (CR), 29.3% are Endangered (EN) and 32.6% of species are Vulnerable (VU). Further, 2.2% of the species are Critically Endangered Possibly Extinct [CR(PE)], 14.1% are Near Threatened (NT) (Ministry of Environment 2012).
The distribution and abundance of orchid populations depend on a suite of biological and ecological factors, including pollinator specialization, seed production and dispersal, limited germination rates, the viability of mycorrhizal fungi (Otero & Flanagan 2006) and appropriate environmental conditions (McCormick & Jacquemyn 2014). Besides, temperature, altitude, and soil pH are the main factors affecting the distribution and abundance of terrestrial orchid species (Djordjević et al. 2016). Sustainability of orchid populations is determined by changes in the number of individuals within a population, the degree of completeness in the ontogenetic spectrum of species, population age and sex structure, etc (Valuiskikh & Teteryuk 2013, Khapugin et al. 2016).
Numerous studies have indicated the importance of certain vegetation types in determining the distribution and abundance of orchids (Landi et al. 2009, Djordjević et al. 2016, Khapugin et al. 2017). Grasslands, wet meadows, bogs, marshes, and montane forests represent important ecosystems that host many orchid species. In Sri Lanka, the lower montane zone (900-1500 m a.s.l.) and montane zone (above 1500 m a.s.l) has recorded the highest wild orchid diversity (Fernando 2013). The measure of the orchid species diversity also provides an insight into the health and the complexity of the ecosystem that they are living in (Fernando 2013, Khapugin et al. 2016).
Among terrestrial orchid species, Ipsea speciosa Lindl. has been identified as an endemic and endangered species (Ministry of Environment 2012) with medicinal properties (Kumari et al. 2006). It is hard to distinguish this species among grasses when there are no flowers as it does not bear leaves during the flowering season. Ipsea speciosa represents a formerly common species, occurring in grasslands of the Central Highlands of Sri Lanka (Jayaweera 1981). However, the number of sites of orchid species including I. speciosa started to decline due to various anthropogenic activities, such as intentional burning, illegal collection from the wild for medicinal purposes etc. (Fernando 2012). Therefore, the present study aimed to determine the population status and the factors affecting the distribution of I. speciosa in isolated populations of unprotected areas in the Central Highlands, Sri Lanka.
Materials and Methods
Study species.—
Ipsea speciosa, popularly called the Daffodil Orchid, is an endemic and endangered terrestrial orchid species occurring in wet grasslands in the Central Highlands of Sri Lanka (Ministry of Environment 2012). It is easily distinguished by its large bright yellow flowers among the grasses of the patana lands in the montane zone (915-1829 m a.s.l). This terrestrial herb has 2-3 cm broadly ovoid pseudobulbs, long filiform roots from their bases. Leaves are usually single, 15-25 x 0.5-2.2 cm, narrowly or lanceolately linear. It produces one or two (rarely three) large, 5.0-6.6 cm across, bright golden- yellow flowers in a tall, erect, sheathed, 15-40 cm long peduncle. The flowering season is from September to February (Jayaweera 1981).
Study sites.—
Information on the possible habitats of I. speciosa was compiled from published information and literature (Jayaweera 1981, Vlas & Vlas 2008, Fernando & Ormerod 2008, Fernando 2013). Based on compiled information, the surveying area was focused on the western slope of the Central Highlands, starting from Nawalapitiya in Kandy district (600 m a.s.l.) to a higher elevation in Ohiya in Badulla district (1850 m a.s.l.). The mean annual temperature of the Central Highlands is 16 °C, with January as the coldest month with respect to mean monthly temperature, and April and August being the warmest months (Department of Meteorology 2019). This region receives relatively high rainfall throughout the year and contains a diversity of edaphic environments, particularly montane forests, tea plantations and grasslands. The grasslands habitats including banks of the streams of the Mahaweli River, abandoned areas in tea (Camellia sinensis) plantations, Eucalyptus plantations, banks and rock outcrops along railway line were investigated.
Based on preliminary survey, there were nine isolated populations of I. speciosa identified. Sites 1-7 formed a single cluster which was located approximately 20 km away from each other in a mid elevational range (800-1200 m a.s.l.) (Fig. 1). The area where the sites 1-7 were located received highest rainfall (above 3200 mm per annum) during the monsoon periods (Department of Meteorology 2019). Site 8 was observed near to human settlement which was located more than 50 km away from other sites, whereas site 9 was observed near to the montane cloud forest (1850 m a.s.l.) (Fig. 1). Both sites were in higher elevation (above 1500 m a.s.l.) in the western slope of the Central Highlands.
Measurement of ecological parameters.—
Two square plots (2m x 2m) were established in each study site to survey the abundance of flowering individuals of I. speciosa during the blooming peak (November- December) in two consecutive years (2017 and 2018). The peak flowering period was determined based on previous literature (Jayaweera 1981, Vlas & Vlas 2008). Each peduncle above the ground was the accounting unit being conditionally treated as an individual for this study. The numbers of flowering individuals in the optimum period of flowering were used for statistical analysis.
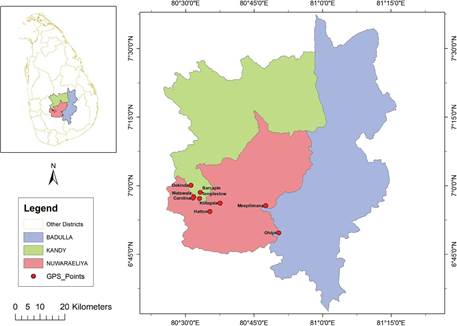
Figure 1 Geographical location of the nine Ipsea speciosa populations found and surveyed at the western slope of the Central Highlands, Sri Lanka.
The vegetation type and the composition of the accompanying flora were recorded in each plot for each site for further interpretation. The relative abundance of accompanying flora was calculated as follows: [Number of individuals of particular species within the two plots / Total number of all individuals of all species in the two plots] × 100. The nomenclature and the threatened status of these species were obtained from The National Red List of Sri Lanka (Ministry of Environment 2012). Recently, several invasive species were observed in the montane ecosystems, especially grasslands habitats; therefore, the abundance of invasive plant species and their potential threats were also recorded. The data set consisted of the species data, and included abundance of I. speciosa, number of accompanying flora, threatened/endemic species, and invasive species. Geographic coordinates in each site were used to make distribution map.
Soil samples were collected 10 cm in depth within the orchid root zone in each plot and bulked. Electrical conductivity (EC) and pH were determined using an EC-meter and a pH-meter in saturation extract, respectively. The percentage of organic matter (OM%) was determined following the protocol of the Department of Agriculture (1997). Total potassium (K) and phosphorus (P) contents were analyzed using the spectrophotometer and flame photometer, respectively. Anthropogenic disturbance such as intentional burning, clearance of vegetation, collection of firewood, lopping grasses for animal feed, among others, were annotated in each site.
Data analysis.—
Study sites were mapped following geographic information system procedures using ArcGIS 10.5. The Shannon diversity index (H’), Simpson index (1-D) and Fisher alpha were used to measure of species abundance, richness and evenness to quantify diversity of the accompanying flora in study sites. Similarity between plant communities in different study sites was explored using the Sorensen Similarity Index (SI). The SI is vary from 0 where the assemblages differ totally to 1 where they are identical (SI = 2c/(a + b), where c is the number of species shared by the two sites, and a and b are the total number of species at each site).
Table 1 Abundance of Ipsea speciosa and status of accompanying flora of nine locations found at the western slope of the Central Highlands, Sri Lanka.
| Site | Locations | Ipsea speciosa | No. of accompanying plant species | No. of endemic/thretened plant species | No. invasive plant species | pH | EC | P (ppm) | K (ppm) | OM (%= | Habitat/ecosytems |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Site 1 | Barcaple | 13 | 17 | 3 | 5 | 5.25 | 10.21 | 8.9 | 77.0 | 4.21 | Grassland near a stream |
| Site 2 | Templestowe | 10 | 15 | 3 | 6 | 5.62 | 16.85 | 11.0 | 98.0 | 4.50 | Grassland in a cemetery |
| Site 3 | Watawala | 21 | 21 | 3 | 7 | 5.43 | 11.01 | 12.0 | 84.0 | 2.43 | Grassland adjoining tea land |
| Site 4 | Caraolina | 3 | 14 | 2 | 4 | 5.41 | 21.51 | 21.51 | 92.0 | 3.25 | Grassland in an Eucalyptus plantation |
| Site 5 | Dekinda | 4 | 13 | 4 | 6 | 5.23 | 11.62 | 111.62 | 74.0 | 2.76 | Banks of railway line |
| Site 6 | Hatton | 23 | 10 | 1 | 5 | 5.12 | 42.20 | 42.20 | 232.0 | 6.07 | Banks of railway line |
| Site 7 | Kotagala | 11 | 14 | 2 | 5 | 5.34 | 24.08 | 24.08 | 114.0 | 4.75 | Banks of railway line |
| Site 8 | Meepilimana | 15 | 7 | 1 | 3 | 5.31 | 25.89 | 25.89 | 123.0 | 5.83 | Grassland in a cemetery |
| Site 9 | Chiya | 2 | 16 | 7 | 4 | 5.20 | 12.05 | 12.05 | 75.0 | 3.34 | Banks of railway line |
A principal component analysis (PCA) and cluster analysis were performed to determine the main contributory factors associated with the grouping of habitats based on vegetation/floristic composition. Ecological parameters such as total number of I. speciosa, accompanying flora, threatened species, invasive species, pH, EC, P, K and organic matter were used for PCA and Cluster analysis. Principal component analysis (PCA) ordination diagram of habitats was generated using first two Principle Components. Statistical analyses were carried out using PAST 3 statistical software.
Results and discussion
All the study sites were located in grassland habitat with various plant communities. There were four sites investigated near the railway line and two grasslands identified as cemetery sites, the rest of the grasslands belong to tea lands, Eucalyptus plantation, and stream bank habitat (Table 1). The abundance of I. speciosa varied from 2 to 23 individuals among nine investigated sites. A large population was observed in site 6 (near Hatton), followed by site 3 (near Watawala), whereas in site 4 (near Carolina) and site 9 (near Ohiya) registered 3 and 2 individuals, respectively (Table 1). In Sri Lanka, several restricted populations of I. speciosa have been documented in protected areas such as Peak Wilderness Protected Area, Horton Plains National Park, and Hakgala Strict Nature Reserve (Kumar & Manilal 1987, Gunatilleke & Pethiyagoda 2012). Unfortunately, all sites investigated during the present study were not entering protected areas.
Accompanying flora.—
The composition of flora accompanying Ipsea speciosa includes 42 species of plants classified in 36 genera (Table 2). The highest number of plant species were recorded in site 3, whereas the lowest numbers were recorded in site 8. Site 3 is adjoining to a tea plantation without large trees. The most species-rich genera were Osbeckia L. (Melastomataceae) with 3 taxa, followed by Mimosa L. (Fabaceae), Panicum L. (Poaceae), and Rubus L. (Rosaceae), with two taxa each. There were six endemic, two endangered, one vulnerable and three near threatened species in the accompanying flora of I. speciosa (Table 2).
Table 2 List of flora accompanying Ipsea speciosa and their relative abundance in each site surveyed at the western slope of the Central Highlands, Sri Lanka. Designations: EN: endangered, VU: vulnerable, NT: near threatened, In: invasive species, * indicate the endemic species.
| Species | Sites | - | - | - | - | - | - | - | - |
|---|---|---|---|---|---|---|---|---|---|
| - | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Ageratina riparia In | - | - | 6.8 | 8.2 | - | - | 13.3 | 38.1 | 14.8 |
| Aristea ecklonii In | - | - | - | - | - | - | 28.6 | 6.2 | |
| Arundina graminifolia | 5.7 | 4.1 | 2.7 | 3.1 | - | 2.6 | 1.9 | - | - |
| Austroeupatorium inulifolium In | 4.8 | 3.1 | 5.5 | 6.1 | 6.6 | 12.8 | 10.5 | 11.9 | 9.9 |
| Blechnum orientalis | 7.6 | - | 6.2 | - | 10.5 | - | 9.5 | - | - |
| Chamaecrista auricoma | - | - | - | - | - | - | - | - | 6.2 |
| Clidemia hirta In | 11.4 | 3.1 | - | - | 11.8 | 12.8 | 9.5 | - | - |
| Clusia rosea In | 8.6 | 10.3 | 8.2 | - | 10.5 | - | - | - | - |
| Crotalaria pallida | - | - | - | - | - | - | 1.0 | - | - |
| Cyanotis thwaitesii NT | - | - | - | - | - | - | - | - | 1.2 |
| Cymbopogon confertiflorus | 11.4 | 11.3 | 12.3 | 15.3 | 13.2 | 15.4 | 13.3 | - | 14.8 |
| Erigeron karvinskianus | - | - | - | - | - | - | - | - | 6.2 |
| Ecucalyptus grandis | 5.7 | - | 3.4 | - | - | - | - | - | - |
| Exacum trinervium*NT | 1.9 | 1.0 | 1.4 | - | - | - | - | - | - |
| Gleichenia linearis | 7.6 | 9.3 | 6.2 | 8.2 | - | 6.4 | 8.6 | - | 4.9 |
| Hedyotis fruticosa | 4.8 | 4.1 | 5.5 | - | - | - | - | - | - |
| Hypochoeris radicata | - | - | - | - | - | - | - | 7.1 | - |
| Ipomoea indica In | - | - | 3.4 | - | - | 5.1 | 3.8 | - | - |
| Lantana camara In | - | - | 4.1 | - | - | - | - | - | - |
| Liparis sp. | - | - | 0.7 | - | - | - | - | - | - |
| Litsea longifolia* | - | - | - | - | 1.3 | - | - | ||
| Lobelia nicotianifolia | 2.9 | 1.0 | - | 4.1 | 5.3 | 6.4 | 4.8 | - | - |
| Lycopodiella cernua | 4.8 | - | 3.4 | 4.1 | - | - | 6.7 | - | - |
| Miconia calvescens In | - | - | - | - | 3.9 | - | - | - | - |
| Mimosa invisa In | - | 8.2 | 6.8 | - | 15.8 | 17.9 | - | - | - |
| Mimosa pudica | - | - | 1.4 | 2.0 | - | - | - | - | - |
| Osbeckia octandra* | 3.8 | 5.2 | 2.1 | 3.1 | 5.3 | - | 2.9 | - | 4.9 |
| Osbeckia parvifolia EN | - | - | - | - | - | - | - | 2.4 | 1.2 |
| Osbeckia rubicunda EN | - | - | - | - | - | - | - | - | 4.9 |
| Panicum maximum In | 4.8 | 10.3 | 7.5 | 15.3 | 11.8 | 16.7 | 10.5 | - | - |
| Panicum repens | 0.0 | 11.3 | 3.4 | 13.3 | - | - | - | - | - |
| Persicaria capitata | - | - | - | - | - | - | - | - | 4.9 |
| Pinus caribaea | 1.9 | - | - | - | - | - | - | - | |
| Pteridium aquilinum In | - | - | - | - | - | - | - | - | 6.2 |
| Rhododendron arboretum* | - | - | - | - | - | - | - | - | 4.9 |
| Rubus ellipticus | - | - | 3.4 | - | - | - | - | 9.5 | - |
| Rubus indicus | - | - | 2.7 | - | - | - | - | - | - |
| Satyrium nepalense NT | - | - | - | - | - | - | - | - | 2.5 |
| Sphagneticola trilobata In | 7.6 | 12.4 | - | 11.2 | - | - | - | - | - |
| Torenia cyanea*VU | - | - | - | - | 1.3 | - | - | - | - |
| Wendlandia bicuspidata* | 4.8 | 5.2 | 2.7 | 6.1 | 2.6 | 3.8 | 3.8 | - | 6.2 |
Table 3 Species richness and diversity indices of different habitats surveyed.
| Parameters | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Site 7 | Site 8 | Site 9 |
|---|---|---|---|---|---|---|---|---|---|
| No. of plant species | 18 | 16 | 23 | 14 | 14 | 11 | 15 | 7 | 17 |
| Individuals | 118 | 107 | 167 | 101 | 80 | 101 | 116 | 56 | 83 |
| Shannon index (H’) | 2.771 | 2.6 | 2.934 | 2.456 | 2.444 | 2.193 | 2.544 | 1.674 | 2.648 |
| Simpson index (1-D) | 0.931 | 0.918 | 0.937 | 0.902 | 0.903 | 0.871 | 0.914 | 0.784 | 0.918 |
| Evenness | 0.887 | 0.841 | 0.817 | 0.832 | 0.823 | 0.815 | 0.849 | 0.762 | 0.831 |
| Fisher alpha | 5.918 | 5.213 | 7.227 | 4.411 | 4.913 | 3.142 | 4.589 | 2.112 | 6.473 |
Table 4 Sorensen index pairwise of the accompanying flora species richness among study sites.
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Site 7 | Site 8 | Site 9 | |
| Site 1 | 1 | - | - | - | - | - | - | - | - |
| Site 2 | 0.813 | 1 | - | - | - | - | - | - | - |
| Site 3 | 0.660 | 0.650 | 1 | - | - | - | - | - | - |
| Site 4 | 0.660 | 0.710 | 0.628 | 1 | - | - | - | - | - |
| Site 5 | 0.600 | 0.640 | 0.457 | 0.461 | 1 | - | - | - | - |
| Site 6 | 0.590 | 0.720 | 0.5 | 0.609 | 0.609 | 1 | - | - | - |
| Site 7 | 0.713 | 0.622 | 0.611 | 0.741 | 0.593 | 0.75 | 1 | - | - |
| Site 8 | 0.087 | 0.095 | 0.143 | 0.211 | 0.105 | 0.125 | 0.2 | 1 | - |
| Site 9 | 0.240 | 0.323 | 0.263 | 0.414 | 0.276 | 0.307 | 0.4 | 0.273 | 1 |
Our results indicate that site 3 is a diverse habitat that maintains the highest species richness, recording 23 species that represent 54.8% of all species recorded in the survey (H’=2.934, Fisher alpha=7.227, Simpson index (1-D)=0.937) (Table 3). Site 1 was the next important habitat recording 18 species (H’=2.771). Site 8 showed the lowest diversity among all habitats (H’=1.674, Fisher alpha=2.112). Several causes could explain variations in the degree of diversity between the sites of the study area: soil physical and chemical properties, rainfall pattern, anthropogenic action, land use pattern, etc. Site 8 was categorized as a cemetery and several anthropogenic activities such as regular slashing, clearing, and burning were observed. Although site 9 was located outside the Horton Plains National Park, the highest abundance of endemic and nationally threatened species was observed in the grassland of this site, which could be due to a less anthropogenic disturbance away from human settlement and restricted movement along the railway line.
Among 41 accompanying flora, only one species (Austroeupatorium inulifolium) was shared among all the sites. Two species (Cymbopogon confertiflorus and Wendlandia bicuspidata) were found in eight sites, except in site 8, while Hypochoeris radicata was unique to site 8. Sites 1 and 2 shared more common species (SI=0.813, Table 4). Site 8 recorded the lowest values for the SI, indicating the most distinct site. Conversely, sites 6 and 8 recorded the lowest richness of threatened species. During the study period, flowering of limited distribution threatened species such as Cyanotis thwaitesii Hassk. (Commelinaceae), Exacum trinervium (L.) Druce (Gentianaceae), and Osbeckia parvifolia Arn. were also observed in few locations.
In addition to the Daffodil Orchid (Ipsea speciosa), three terrestrial orchid species were observed at the study sites. Arundina graminifolia (D.Don) Hochr. (Fig. 2B) was commonly found at sites 1-7 in mid-elevation, except site 6. Satyrium nepalense D.Don (Fig. 2C) was recorded only in site 9 with a few individuals. One orchid species observed in site 3 without flowers and pods, may belong to the genus Liparis Rich. based on leaf characters. The results of the principal component analysis (PCA) (Fig. 3) and hierarchical cluster analysis (Fig. 4) exhibited that the selected vegetation parameters strongly affect the grouping of sampled grasslands. The first two principal components (PCs) of floristic compositions of investigated sites account for 99.3% of the total variability (Fig. 3). The total number of accompanying plants and the abundance of I. speciosa are the most contributing factors for the clustering of habitats. Following both, the principal component analysis (PCA) and hierarchical cluster analysis (Fig. 4), locations studied were grouped into three main clusters. Sites 1-5 located within 100 km2 and the rest of the sites are scattered geographically.

Photographs by C. Mahanayake (A) and J. D. Kottawa-Arachchi (B-C).
Figure 2 A. Blooming of Daffodil Orchid, Ipsea speciosa, in grassland. B. Arundina graminifolia blooming at site 2. C. Satyrium nepalense blooming at site 9.
Out of the nine locations, sites 7 and 8 grouped in 1st cluster. Cluster II comprised six sites (1, 2, 3, 4, 5 and 9) and biplot showed scatter distribution of those sites suggesting the distance relationship each other. Sites 4, 5 and 9 which are representing few individuals of I. speciosa and critical conservation measures should be required to protect those small populations. Only site 6 is separate from the rest of the sites representing a relatively higher number of I. speciosa and accompanying species. The grassland habitats in two sites (5 and 9) recorded higher threatened species. These sites are about 2-3 km away from human settlements which could consider as less disturbed sites than the rest of the locations. The clusters representing a close association in biplot could be explained by similar vegetation communities and positions within clusters.
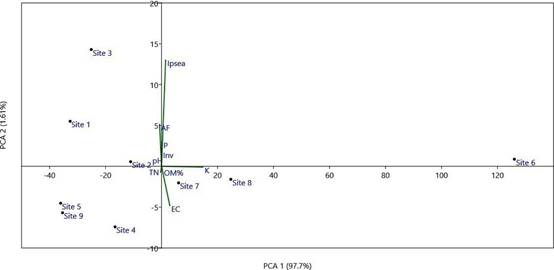
Designations: Ipsea: abundance of I. speciosa, AF: total numbers of accompanying flora, TN: endemic/ threatened species, Inv: invasive species. EC: electric conductivity, P: phosphorus content, K: potassium content, OM: organic matter.
Figure 3 Principal component analysis (PCA) ordination diagram of sites with presence of I. speciosa based on vegetation parameters.
Factors determining distribution and abundance of Ipsea speciosa.—
The results of this study show that soil properties such as EC, K, and P effectively influence the distribution and abundance of orchid species within sampled grasslands. The pH of soil samples varied from 5.12 to 5.62. Organic matter (OM) content on the soils investigated was between 2.43% and 6.07% (Table 1). The OM was higher than 3% for 7 sites. Therefore, much of the investigated soils of orchid growing areas could be classified into high organic matter soils. Djordjević & Tsiftsis (2020) reported that a significant number of species of orchids that prefer acidic soils grow in high-altitude areas. Tsiftsis et al. (2008) revealed that altitude represents a complex variable related to climatic factors and it is positively correlated with the organic matter content of the soil. Furthermore, they mentioned that soil acidity was the most important factor in determining orchid distribution. The EC values of the soils were found to be between 10.21 dS m-1 and 42.20 dS m-1. The highest K concentration (232.0 ppm) was detected in site 6, followed by sites 8 and 7. Regular intentional fires occurring at sites 4, 6, 7 could be the reason for detecting the high concentration of K in the soil.
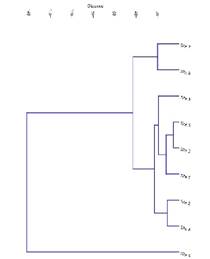
Figure 4 Hierarchical cluster analysis of nine locations based on ecological parameters (total number of I. speciosa, accompanying flora, threatened species, invasive species, pH, EC, P, K and organic matter).
The results of this study show that vegetation type and soil properties effectively influence the distribution and abundance of I. speciosa within sampled grasslands. Similarly, Tsiftsis et al. (2008) found that the most important gradients that govern orchid distribution in northeastern Greece are altitude, soil acidity, and specific habitat types. Furthermore, recent studies suggested that orchid richness is regulated by habitat size, altitude, and climate at large scales (Acharya et al. 2011, Zhang et al. 2015).
Although very little has been published on the life cycle of I. speciosa, Kumari et al. (2006) reported the successful artificial propagation of seeds under in vitro conditions. According to the report, it takes approximately 10 weeks for a fruit/capsule to mature to the point when the seeds are brown, and then take 60 days to germinate. The germination period would be altered due to particular weather factors such as temperature, moisture, etc. Furthermore, it is challenging to locate seedling orchids in dense vegetation, yet identifying the presence of early plant stages (such as protocorms) in soil subplots near mature plants is critical for a future demographic investigation.
Threat and conservation.—
Habitat deterioration and degradation, clearing of vegetation, intentional forest fires, and the spread of invasive alien species are significant threats to highland grasslands and wetlands in Sri Lanka (Kotagama & Bambaradeniya 2006, Ministry of Environment 2010). In the montane and submontane areas, forests and grasslands are cleared for vegetable cultivation, this being the main agriculture- based threat. The excessive use of agrochemicals is believed to have a considerable impact on the survival of the orchid populations. Besides, forest felling for firewood, encroachments and illegal settlements, intentional forest fire, and garbage dumping are the main habitat-related threats to native orchid survival (Fernando 2012).
With the increasing demand for agricultural products, abandoned lands, especially grasslands, are vulnerable to encroachment. Unfortunately, these encroachments are established permanently, leading to significant loss of natural vegetation (Kottawa- Arachchi 2017). Furthermore, fragmentation of habitats also has a detrimental effect on small populations, especially rare and threatened species.
Spreading of invasive alien species.—
Invasive alien plants have been widely recognized to exert a significant negative impact including superior competitors for limited resources in comparison with their native counterparts (Vila et al. 2011). In addition to the native flora, 12 invasive plant species were recorded as accompanying flora. The highest numbers of invasive species were observed in site 3 (near Watawala). Among the invasive species, the Neotropical shrub Austroeupatorium inulifolium (Kunth) R.M.King & H.Rob. (Asteraceae) was recorded at all the sites, and Panicum maximum Jacq. (Poaceae) was observed at sites 1-7 as dominant or common species. During the survey, we observed rapidly growing invasive species Ageratina riparia (Regel) R.M.King & H.Rob. (Asteraceae) and Mimosa invisa Mart. ex Colla (Fabaceae) populations in five and four locations, respectively (Table 2). Numerous studies have shown that invasive plant species impose greater effects than dominant native species on the growth and reproduction of native residents (Vila et al. 2011, Kuebbing & Nunez 2016). The presence of invasive species has a negative effect on native plants, indicating that invasive species may displace native species such as the terrestrial orchids Ipsea speciosa, and Satyrium nepalense. The rapid spreading of A. riparia and P. maximum was observed in site 8 and site 3, respectively. The current growth of the invasive species mentioned above is indicative of the future shrinkage of the populations of native terrestrial orchids. In addition to orchids, many threatened grassland species such as Exacum trinervium (L.) Druce (Gentianaceae), Osbeckia parvifolia Arn., and Torenia cyanea Atton (Linderniaceae) could be lost because native plants are unlikely to be self-sustaining in the competition with invasive plants.

Figure 5 Set fires in Kotagala site. Photo by J. D. Kottawa-Arachchi, captured on 26th December 2017.
Habitat deterioration.—
Fire disturbance is serious when soil is overheated, and most of the grasslands in Central Highlands set fires during the dry period, especially from January to April. Incidences of intentional fires in study sites, especially Watawala, Hatton, and Kotagala, have been observed in past decades. Unfortunately, set fires were observed in Kotagala (Fig. 5) and Hatton sites after this study. Repeated burning might be deleterious and could also negatively impact existing protocorms, seedlings, and non-flowering mature plants, as well as the reproductive parts such as flowers, capsules, and seeds, and could even alter the mycorrhizal community, hence, lead to the loss of terrestrial orchids and native flora from their habitats. Out of nine habitats, four populations were recorded at banks of railway lines which are regularly cleared by the workers of the Department of Railwayv. This could be a negative impact on restricted populations. Therefore, immediate and continuous management is required to prevent further loss of remnant populations of I. speciosa in such valuable grasslands.
Orchids with showy flowers and medicinal properties encounter an added disadvantage due to over- collection from the wild. Ipsea speciosa is subjected to removal from the wild for medicinal purposes and due to various mythological beliefs connected to its tuber (Fig. 6) (Fernando 2012). Kumari et al. (2006) developed an in-vitro protocol for mass propagation of the species and rhizome tips were found to be suitable as a source of explant. This suggested approach could be useful to generate an adequate number of plants hence minimize the illegal collections from the wild.
Climate change impacts the environment leading to a reduction in the distribution and abundance of species, especially endemics, which may even result in their global extinction. Climate change can also affect biota of the various ecosystems through extreme rains, changes in evaporation, modification of the microclimate, and alteration of the composition of forest-grassland flora (Reina-Rodriguez et al. 2017).
Although grasslands are considered as vulnerable to climate change, the long term monitoring of adaptability of plant communities and reproductive biology, which respond to extreme weather events, is essential for understanding the future impact of climate change (Kottawa-Arachchi & Wijeratne 2017).
Conclusions
The present study demonstrates that the Daffodil Orchid, I. speciosa, shows a restricted distribution in the Western slope of the Central Highlands, Sri Lanka. Its presence is confined to a few grassland habitats where increasing anthropogenic activities have been observed. Based on the spreading of invasive species, all sites are vulnerable to shrinkage of terrestrial orchid populations, including I. speciosa. Besides, intentional firing is another serious threat to the grasslands, and these anthropogenic activities affect negatively the native grassland flora, including terrestrial orchids. The present study revealed that habitat conservation is an important strategy for the protection of threatened species. To better understand the population dynamics of I. speciosa, our results suggest continuing monitoring and assessment of its population in selected grasslands from Sri Lanka.












 uBio
uBio 


