Introduction
The Xenarthra are characterized by a particular suite of skeletal modifications, e. g. shape and relationship of the vertebral metapophysis on the last thoracic and lumbar vertebra, presence of synsacrum, isquio-sacral union, sacroischiatic fenestra, reduction or absence of enamel, monophiodonty and hypsodonthy (McDonald, 2003)[1]. The Xenarthrans constitute one of the most peculiar and characteristic clades of South American mammals (Pujos, et al., 2013)[2]. Xenarthra, including fossil and living species, consist of two major clades: Pilosa (Sloths, and Antearters), without dermal armor, but covering with a dense hair, which comprise Vermilingua and Eufolivora, and Cingulata (Armadillos, pampatheres and glyptodonts), characterized by the development of bony dermal armor (armoured Xenarthra) (Carlini, Brandoni & Molin, 2013 [3]; Pujos & De Iuliis, 2007 [4]; Pujos, et al., 2013 [2]; Varela,Tambusso, McDonald & Fariña, 2019 [5]).
The last hypotheses of the Eufolivora or Sloths phylogenetic relationship, based on bayesian morphological clock analysis, are grouping they in five major clades: Mylodontidae, Nothrotheriidae, Megatheriidae, Megalonychidae and Bradypodidae (Varela et al., 2019) [5].
The Megatheriidae are small to giant ground sloths, and they are diagnosed by eight unequivocal synapomorphies. These include an elongate mandibular symphysis and spout [62(3) and 68(2), Fig. 8 (Gaudin, 2004) [6]], the lack of a clear demarcation between the posterior end of the symphysis and the ventral edge of the horizontal ramus of the mandible in lateral view [69(1), Gaudin, 2004][6].
Dental characters have been of particular importance in dividing the diverse assemblage of extinct ground sloths into its traditional constituent families: the Megatheriidae, typified by large square teeth with parallel transverse crests and a thickened external layer of cementum; the Mylodontidae, diagnosed by their peculiar lobate dentition; and the Megalonychidae, identified by their quadrate or ovate molariform teeth with subparallel crests, and by their enlarged caniniform or incisiform first upper and lower teeth (Gaudin, 2004)[6].
In addition, the three auditory region synapomorphies of the Megatheriidae identified by Gaudin (1995) [7] also serve to diagnose this node. The sister-group relationship between Eremotherium and Megatherium (subfamily Megatheriinae, Node 25) is one of the most robust nodes on the cladogram. It is diagnosed by 48 unambiguous synapomorphies, among them nine unique to the megatheriines. Of these nine, seven are dental characters: the third lower molariform is the smallest molariform; the upper caniniform is square in cross-section; the lower caniniform is rectangular in cross-section; the lower first molariform is square in cross-section; the second and third upper molariforms are square in cross-section; the second lower molariform is square in cross-section; and the third lower molariform is trapezoidal in cross-section.
The other two include a posterior external opening of the mandibular canal that faces anteromedial, lying on the internal surface of the ascending ramus, and a lacrimal foramen that opens into a ventrally directed canal on the surface of the lacrimal bone (Gaudin, 2004)[6].
The megatheriidae include two Subfamilies: Planopsinae and Megatheriinae (Varela, et al., 2019)[5]. Overall, the list of valid megatheriine genera from the Middle Miocene–Pliocene of South America currently comprises Megathericulus Ameghino, (1904), Eomegatherium Kraglievich (1926), Promegatherium Ameghino (1883), Megatheridium Cabrera (1928), Pliomegatherium Kraglievich (1930), Megatheriops Ameghino and Kraglievich (1921), Plesiomegatherium Roth (1911), Megatheridium Cabrera (1928), Pyramiodontherium Rovereto (1914), Anisodontherium Brandoni & De Iuliis (2007) and Brandoni, Powell & González (2012), Urumaquia Carlini, Brandoni & Sánchez (2006) and Proeremotherium Carlini et al. (2006).
There are also megathere fossils from the Middle Miocene of Cerdas, Bolivia, reported as Megatheriidae indeterminate (Croft et al., 2016)[8]. Hirschfeld (1985)[9] reported some material of indeterminate Megatheriinae from the middle Miocene of La Venta in Colombia.
Some features in the skull of the Pliocene (Codore Formation, Urumaco desert Falcon state Venezuela) taxa Proeremotherium eljebe could indicate an ancestral or close relationship with the geographically-widespread megatheriine Eremotherium laurillardi (Carlini et al., 2006)[10].
Eremotherium comprises three species: E. eomigrans De Iuliis and Cartelle, 1999, from the late Pliocene (Blancan) of the USA; E. sefvei De Iuliis and St-André, 1997, from the Pleistocene of Bolivia, and E. laurillardi from several late Pleistocene localities of North America (e.g., southern Mexico and west coast of USA up to South Carolina), Central America and low latitudes of South America (De Iuliis, 1996)[11].
The only one report of a Megatheriidae in the late Miocene of Central America was made by Laurito and Valerio (2012a)[12], who reported some teeth of Megatheriidae genus and species indeterminate, from San Gerardo de Limoncito, Curré Formation, (Early Hemphillian Hh2 - Late Miocene) from Costa Rica. Also, a juvenile specimen of Pliometanastes has been reported from that locality by Valerio and Laurito (2014)[13], very similar to? Pliometanastes galushai, which is why they considered the specimen as a juvenile of Pliometanastes sp.
Age, geology and locality
The rock unit that contains the fossiliferous remains corresponds to the Curré Formation. Henningsen (1965)[16] defines this formation as a series of tuffaceous sandstones with intercalations of conglomerates, siltstones and occasionally shales, dark green and brownish green when altered. Mora (1979)[15], describes two subunits according to their sedimentological characteristics: The subunit of volcaniclastic conglomerates of gray-green color, overlain by shales that settled in a transitional environment between delta and beach. The subunit of black shales, interspersed with strata of sandstones and fine conglomerates, was deposited in a paralic environment of estuary or swamp type. They are very chloritized and basically consist of subvolcanic products.
The paleontological material recovered is housed at the Geology Section of the Natural History Department at The National Museum of Costa Rica, and comes from a level of fine conglomerate that is located towards the roof of a sequence of blue clays, which in turn is overlain by a sequence of medium to fine sandstones, which confirms its association with the superior subunit or subunit of shales (sensu Mora, 1979)[15].
Granados and Aguilar (1983)[14], based on the presence of molluscs, obtained a Middle-Late Miocene age for the Curré Formation.
With regard to sedimentology, in the San Gerardo de Limoncito locality the following sedimentary facies are established (Figure 1, Figure 2).
Neritic or Proximal Platform
The lithofacies of glauconitic blue clay characterizes this depositional environment and suggests a slow sedimentation rate. This lithofacies is constituted by a bank of 2 meters of visible thickness, of decimetric strata of clays and silts, finely laminated with abundant plant detritus and wood reworked by the waves, and floating wood perforated by Teredo sp. No other fossil traces are observed into the sediments, which, together with the abundant presence of glauconite and carbonaceous material, is interpreted as a predominantly reducing environment (Chafetz & Reid, 2000)[17].
Subaquatic fan delta
The lithofacies of conglomerates is what characterizes this depositional environment and is composed of blue clay intraclasts, coming from the lower level which it overlies at an erosional discordance. The clasts have diameters that vary between 5 and 13 cm; they are well rounded, little or not spherical; some have point contact, but 90% of them float in a matrix of glauconitic clay. The clasts are arranged with their long axis parallel to the layer of stratification, and it is possible to observe some degree of imbrication. In this sequence are frequent bone and dental remains of various vertebrates, both terrestrial and marine. The Trypanites ichnofacies, in this case, is inferred indirectly from the blue clay intraclasts that are usually perforated by bivalves of the family Pholadidae, from which it is still possible to find remains of the valves within the fossil traces of Gastrochaenolites sp.
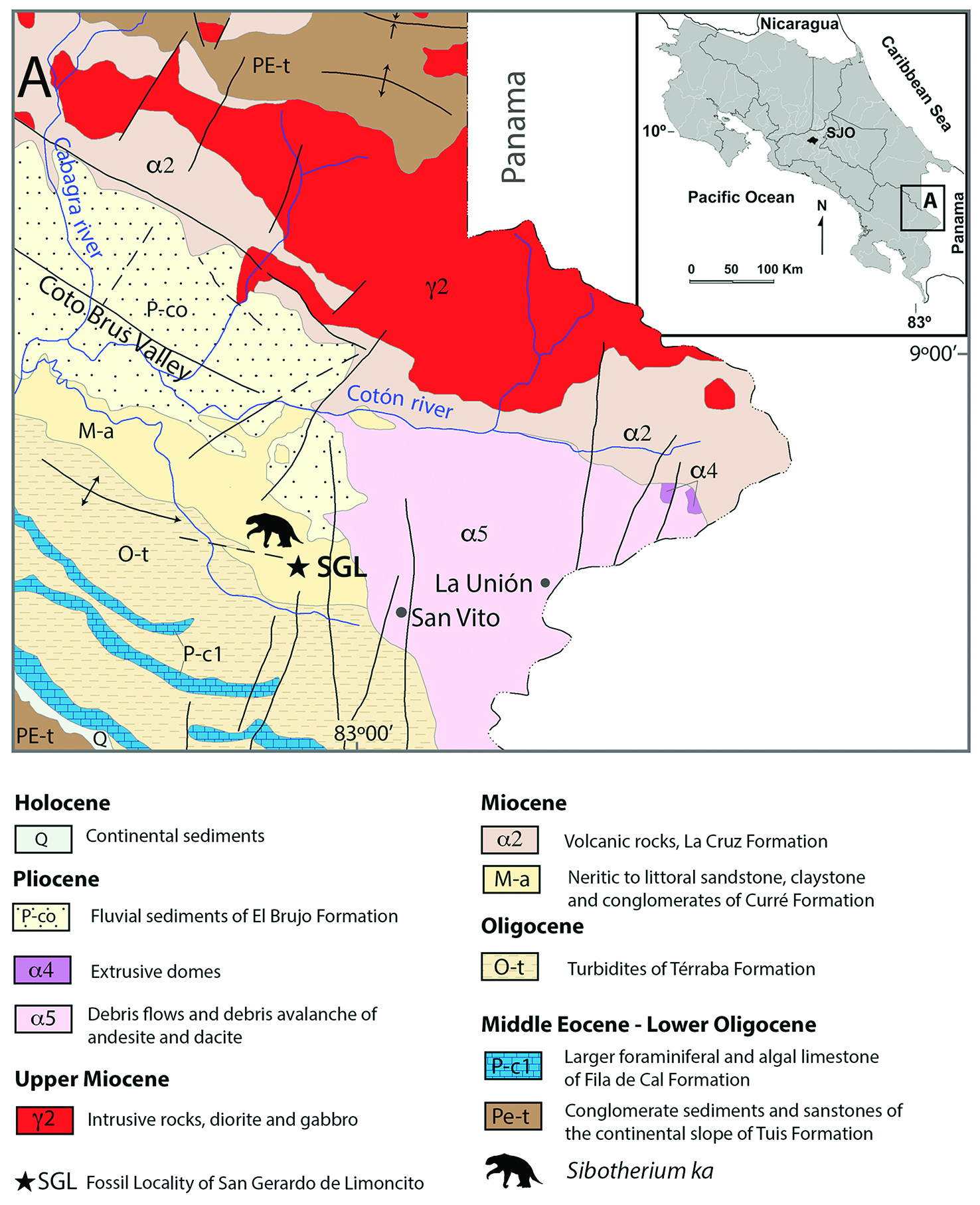
Figure 1 Geographic location of studied area, Geological map of the San Gerardo de Limoncito area, modified from Denyer & Alvarado (2007)[18].
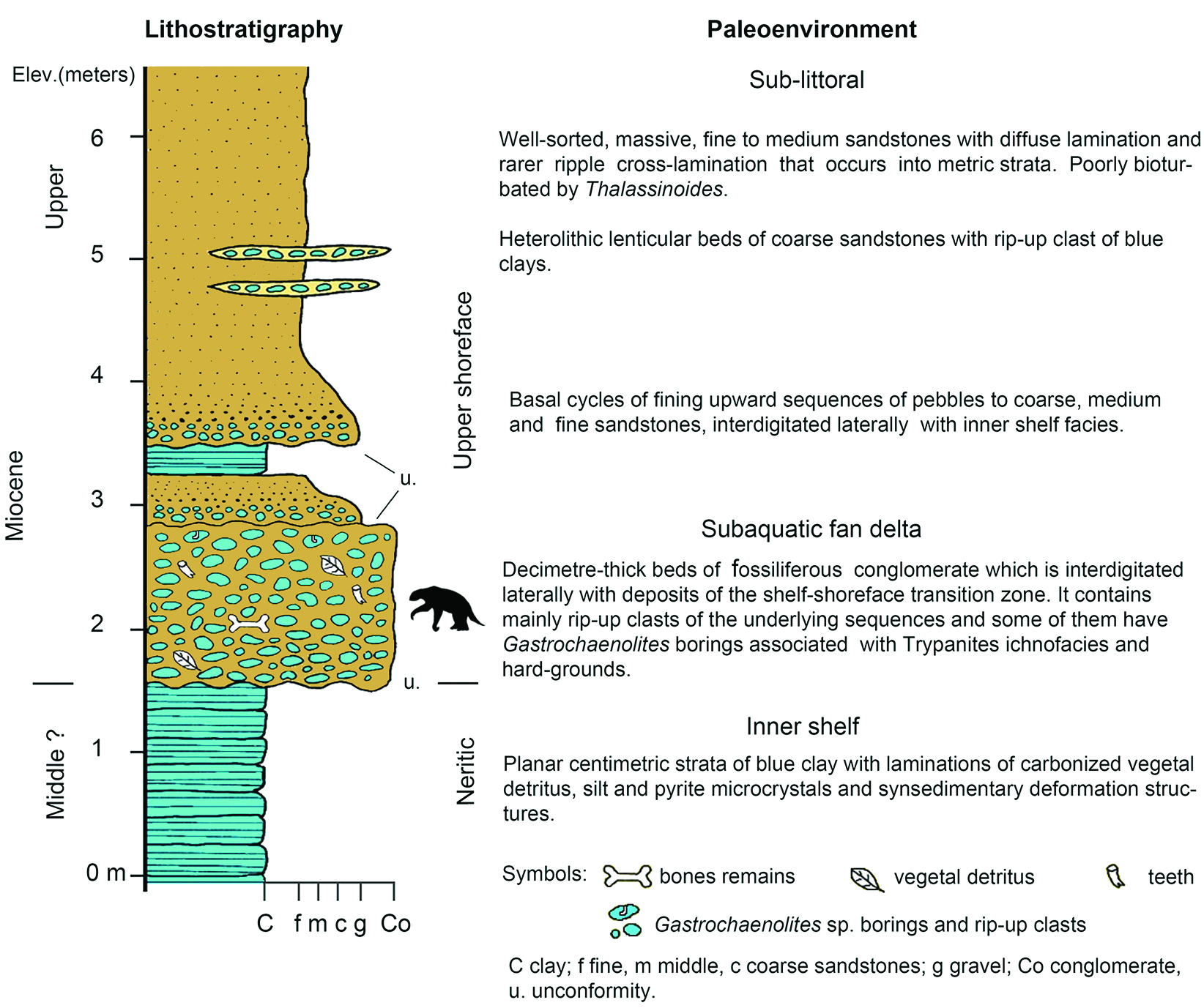
Figure 2 Stratigraphic column and sedimentological interpretation of the locality of San Gerardo de Limoncito, Costa Rica (Laurito & Valerio, 2010)[19] where Sibotherium ka gen. et sp. nov. was found.
This suggests the existence of lithified bottoms in marine environments at depths not greater than 35 m, suggested by the Pholadidae traces and associated with rocky coasts, hardgrounds, unconformities and other surfaces of omission; this is also confirmed by the presence of oysters and parautocthonous Cirripedia, as well as the shell of a male crab identified as Callinectes sp. However, it is clear that these underwater, shallow, hardgrounds were located in areas adjacent to or directly below where the sedimentary lobes of the subtidal fan delta, associated with a fluvial system, were deposited. The energy of the deposit was associated with one or several periods of rainfall, which reached the traction necessary to erode the hardgrounds and produce the “rip up clasts” or intraclasts, and even to detach fragments of the blue clays and integrate them within the thick sediments of the fan delta.
Infralitoral
The lithofacies of fine-to medium-grained sandstones, well selected and clean, is the predominant lithology and forms a bank of 2.5 m thickness, constituted by metric and homogeneous strata in which a diffuse parallel lamination and low angle foresets associated with small tidal channels is observed. Other levels present scarce carbonaceous material and infrequent ichnofossils. This sequence overlaps the conglomerate facies by a sedimentary discordance and presents two centimeter cycles of coarse gravels at its base that quickly grade to medium and fine sands. Also, it was verified in the field that this sequence interdigitates laterally with the facies of the internal platform, since it was observed that these fine sands partially reach the blue clay facies, directly overlying it at some points. The Skolithos ichnofacies, in this case, is deduced from small and scarce traces of Thalassinoides sp., suggesting a high energy environment, associated with clean and well selected sediments with parallel lamination and low angle foresets. This is in accordance with the definition given by Seilacher (1967)[20] for this ichnofacies.
Systematic paleontology
Xenarthra Cope (1889)
Phyllophaga Owen (1842)
Megatherioidea Gray (1821)
Megatheriidae Gray (1821)
Megatheriinae Gray (1821)
SibotheriumGen. Nov.
DIAGNOSIS. — Sibotherium differs from other Megatheriinae in the following character combination: Large size Megatheriidae-Megatheriinae; the posterior end of the symphysis lies well anterior to the first molariform; the m4 is posterior to the anterior margin of the ascending ramus, and not visible in lateral view; molariforms 6th to 22 % wider than long; the valleys between the lophs of the occlusal surface of the molariforms are V-shaped and shallow; astragalus 12,5% wider than long than any other medium side Megatheriinae; the navicular facet lies dorsally, with plane of discoid facet intersecting it approximately at the middle of the navicular depression; navicular facet lies posterior to the most anterior border of discoid facet, in dorsal view; odontoid process and odontoid facet lateral to the navicular facet; Navicular facet projects medially more than in any other Megatheriinae; sulcus talis most mesial, and short; the odontoid process is 110º from the discoid facet.
ETYMOLOGY. — Sibö, Bribri world from Talamancan Mythology means good creator, and the Greek Therios for beast, meaning the beast of Sibö, in allusion of the first mammals appearing before the connection of the Americas. Cf. Jara Murillo, C. V. 2003. Diccionario de Mitología Bribri. Edition: 2.ª reimp.(2014), 336 pp[21].
TYPE SPECIES. — Sibotherium ka gen. et sp. nov.
Sibotherium kagen. et sp. nov.
2012. Megatheriidae indeterminate. Laurito & Valerio (2012a)[12]
DIAGNOSIS. — As for the genus
ETYMOLOGY. — Ká from Bribri mythology, which mean site, referred to the Coto Brus Valley, the site of Sibö, Sibotherium ka, means the Beast of the site of Sibö. In the Bribri Myth, Sibö created in the beginning the earth and main land, and all the animals and plants. The new Megatheriinae from Coto Brus Valley, in Costa Rica, Central America, symbolizes one of the first South American Heralds that appeared previous to the closure of the Panama Isthmus.
HOLOTYPE. — CFM-994, left astragalus.
Referred material— CFM-2607, posterior fragment of a right mandible, just with the posterior part of the alveolus for m4, and the base of the coronoid process; CFM-3731, most anterior part of a mandible with the most anterior part of alveolus for m1; fragments of molariforms with the occlusal surface, CFM-3634, 3000, 1532, 1530, 1447, 1858; fragments of molariforms, CFM-1531, 2604, 2606, 1696; CFM-1654, left astragalus, lacking the odontoid process, and the most dorsal part of the navicular facet; CFM-993, right astragalus lacking the odontoid process, navicular process, and sustentacular facet;CFM-3813, fragment of navicular process of right astragalus; CFM-995, right calcaneum without the sustentacular facet and the posterior half of tuber calcis; CFM-998, left tuber calcis of calcaneum; CFM-1243, fragment of calcaneus just with the octal facet; CFM-1239, proximal part of right fourth metatarsal; CFM-1301, distal part of fourth right metatarsal; CFM-2498, proximal part of fifth metatarsal; CFM-1645, left navicular; CFM-3058, right navicular of young individual; CFM-3795, clavicle; CFM-1840, proximal part of right tibia; CFM-1302, fragment of lateral trochlea of right tibia; CFM-2625, left magnum; CFM-1785, left lunar; CFM-3713, left lunar.
TYPE LOCALITY. — Curré Formation, San Gerardo de Limoncito, Costa Rica.
AGE. — Late Hemphillian (Hh3) (Late Miocene), Curré Formation, San Gerardo de Limoncito, Costa Rica. 87Sr/86Sr 5.80 Ma +/- 0.60/-0.80 (Table 1).
Table 1 Age of Curré Formation, San Gerardo de Limoncito, Costa Rica.
| Sample | Lab code | Type of material | 87 Sr/ 86Sr | 1 sd* | 2SE(M)* | n | Weight grams | Ma | ±Ma |
| CR-1 | 6058 AR CR | Oyster | 0.709004 | 28 | 7 | 59 | 0.05857 | 5.80 | 0.60/-0.80 |
| CR-2 | 6059 AR CR | Mollusc | 0.708854 | 25 | 7 | 57 | 0.06235 | 11.15 | 1.55/-1.10 |
Sr isotope analyses were performed with a THERMO SCIENTIFIC TRITON PLUS Thermal Ionization Mass Spectrometer (TIMS).
Laboratory NBS987 standard: 0.710253 ± 12* n = 81
1 sd = 1 standard deviation; *) in the last two figures
2 SE (M) = 2sd / root n
n = number of relationships measured per run
Ages calculated with: LOWESS 5 Fit 26 03 13
McArthur, J.M.; Howarth, R.J.; Shields, G.A. 2012. Strontium Isotope Stratigraphy. In: The Geologic Time Scale Vols 1 & 2 Pages: 127-144; Gradstein, FM; Ogg, JG; Schmitz, MD; et al. (Eds).
Descrption
Molariforms and mandible. — The molariforms (Figure 3) in Sibotherium are slightly larger than in Anisodontherium; the valleys between the transverse crests in the molariforms of Sibotherium are shallow but V-shaped, as in Pyramiodontherium, Megatheriops, Eremotherium or Megatherium, but the Anisodontherium molariforms have a markedly deep V-shaped valley between the transverse crests, particularly in m1 (Brandoni & De Iuliis, 2007[22]; Brandoni et al., 2012[23]; Saint-André & De Iuliis, 2001)[24].
The Sibotherium molariforms are mesiodistally compressed, and rectangular in section rather than squared, so they are 6 to 22 % wider than long, but they are less compressed mesiodistally than Anisodontherium (Brandoni et al., 2012)[23], where are extremely compressed (13 to 37% wider than long), Megathericulus patagonicus (38% wider than long) (Brandoni et al., 2017)[25], Eomegatherium andinum, or La Ventan Megatheriidae. This is in marked contrast to the nearly squared molariforms present in all other known megatheriines, such as Pyramiodontherium, Megatheriops, Megatherium, Eremotherium, Plesiomegatherium hansmeyeri, Pliomegatherium lelongi and Proeremotherium (Brandoni & De Iuliis, 2007[22]; Carlini, Brandoni, Sánchez & Sánchez-Villagra, 2018[26]). The teeth of Sibotherium have a V-shaped occlusal surface, but not so deep or highly rectangular as in Anisodontherium (Brandoni et al., 2012)[23]. In Proeremotherium eljebe M1–4 are essentially as wide as long, and M5 is longer than wide (Carlini et al., 2006)[10], however, the measurements reported by Carlini et al. of the teeth reveals a tendency to be 3 to 38 % longer than wide, and differs from Sibotherium in which the teeth are wider than long and thus rectangular in shape.
The first upper molariform (CFM-1530) is trapezoidal in occlusal view, with the lingual and labial wall of the tooth oblique with respect to the midline of the tooth. The mesial loph is narrower and higher than the distal loph, which is longer and very low.
The M1-M2 (CFM-3000; CFM-3634), are slightly rectangular, or nearly quadrangular, 6.5 and 6.9 % wider than long, with the lophs equal in size and at the same level.
The first right lower molariform CFM-1858 of Sibotherium is trapezoidal, but rectangular (17% wider than long) in occlusal view, with the mesial or anterior loph narrow, highly oblique with respect to the lingual wall and slightly higher than the posterior loph, which is wider than the mesial, perpendicular to the lingual wall of the tooth, low than mesial, and both lophs are less sharp than in Eremotherium laurillardi. The lingual wall of the first molariform is parallel with respect to the midline of the tooth, but the labial wall is very oblique, and resembles the first lower tooth of Eremotherium and Megatherium (De Iuliis, 1996)[11].
The second lower molariform of Sibotherium (CFM-1532 right) resembles the first lower molariform in its morphology, but the mesial loph and the labial wall of the tooth is less oblique than the first one. However, it is one of the most compressed teeth of Sibotherium being 22% wider than long, but is less compressed than Anisodontherium in which m2 is 32.14% wider than long (Brandoni et al., 2012)[23].
The third lower molariform (CFM-1447 right) is rectangular in shape in occlusal view, 13.8 % wider than long, and less compressed than Anisodontherium in which is 34.6% wider than long.
In the anterior fragment of the mandible of Sibotherium (CFM-3731) the posterior margin of the mandibular symphysis lies well anterior (antero-posteriorly) to the first molariform, and approximately in the same position as in Megathericulus patagonicus, Anisodontherium halmyronomum and Eomegatherium andinum (according to the characters indicated by Brandoni et al., 2012[23]). In other megatheriines for which this character is known, the symphysis ends more posteriorly than the first molariform, reaching at least to the level of the mesial margin of m1 and extending, as in Eremotherium, or Megatherium americanum, as far back as the middle of m2 (Pujos & Salas, 2004[27]; Saint-André & De Iuliis, 2001[24]). In Pliomegatherium lelongi and Eremotherium laurillardi the symphysis reaches at least at middle to the m1.
In Plesiomegatherium hansmeyeri the posterior border of the mandibular symphysis lies at the level of the alveolar septum between m1 and m2 (Brandoni, Carlini, Anaya, Gans & Croft, 2017)[25], and in Sibotherium the symphysis is well anterior to m1. However, Pujos & Salas (2004)[27] suggested that posterior end of mandibular symphysis in Plesiomegatherium hansmeyeri is anterior to m1.
In Sibotherium the end of the mandibular symphysis starts above the most ventral end of the root of m1, differing from Eremotherium laurillardi where the end of mandibular symphysis starts at the same level of the most ventral end of the m1.
In Sibotherium the mandibular symphysis is very vertically positioned with respect to the anterior border wall of m1 about 30º, as in Pliomegatherium lelongi (Brandoni, 2006, 2013)[28, 29], differing from Eremotherium laurillardi in which the mandibular symphysis is about 60º with respect to the anterior border wall of m1. Besides, in Sibotherium the mandibular symphyseal spout is shorter than in any Megatheriidae in which the mandible is preserved.
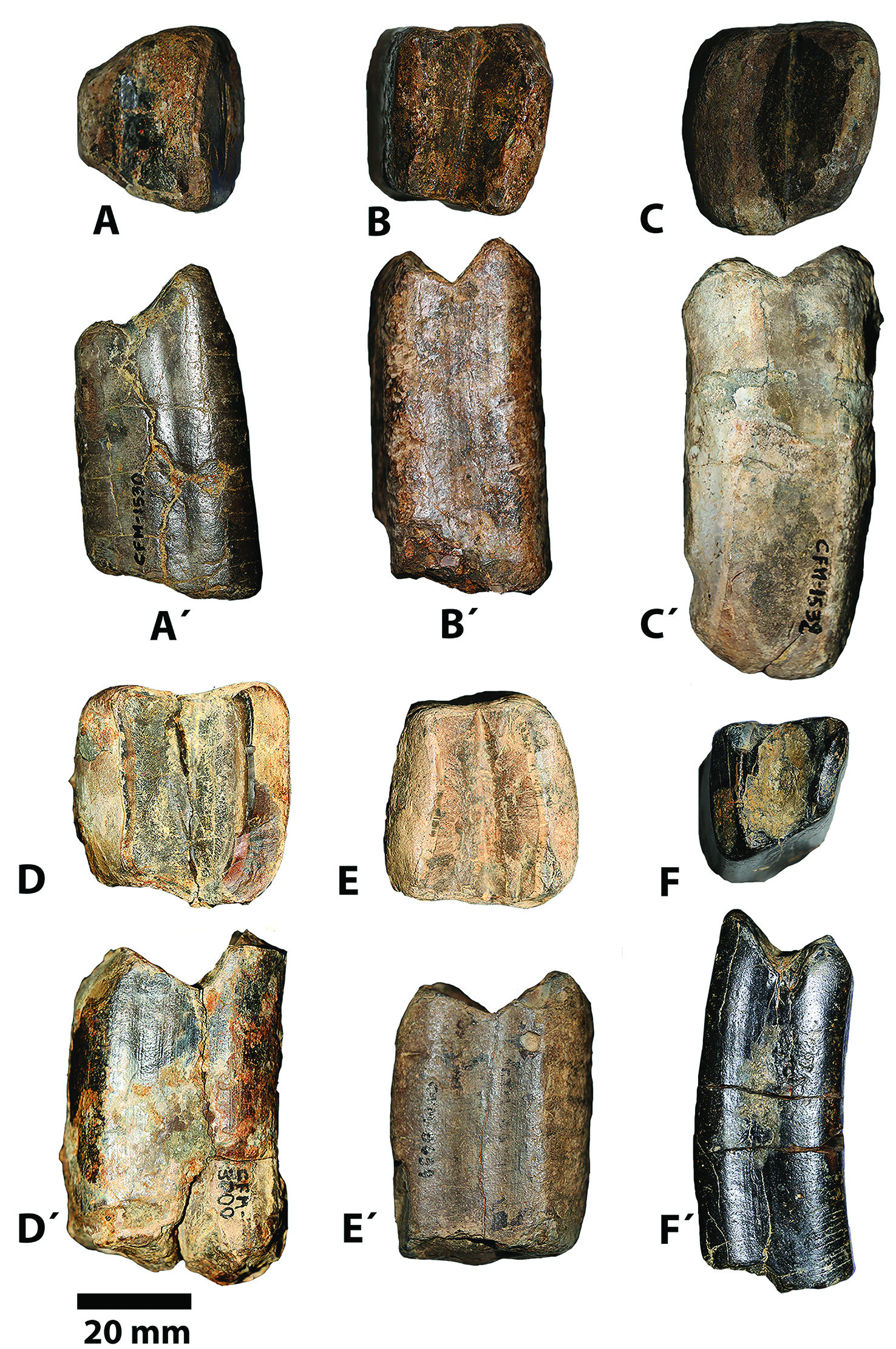
Figure 3 Molariforms of Sibotherium ka gen. et sp. nov. in occlusal view A. CFM-1530, first upper molariform, B. CFM-1447, third lower molariform, C.CFM-1532 second lower molariform, and D. CFM- 3000 first or second upper molariform, E. CFM-3634, first or second upper molariform; F. CFM-1858 first lower molariform), and labial views (A´, B´, C´ and D´, E´, F´).
In Megathericulus patagonicus, Pliomegatherium lelongi, Eremotherium laurillardi, Megatherium americanum, Megatherium altiplanicum, Anisodontherium sp. the symphyseal spout is prominent and long (Brandoni, 2006, 2013[28, 29]; Brandoni et al., 2012[23]; De Iuliis et al., 2008[30]; Saint-André & De Iuliis, 2001[24]).
In Sibotherium the m4 is posterior to the anterior margin of the ascending ramus, and not visible in lateral view. In Anisodontherium the m4 is anterior to anterior margin of ascending ramus, so that it is visible in lateral view (Brandoni et al., 2012)[23].
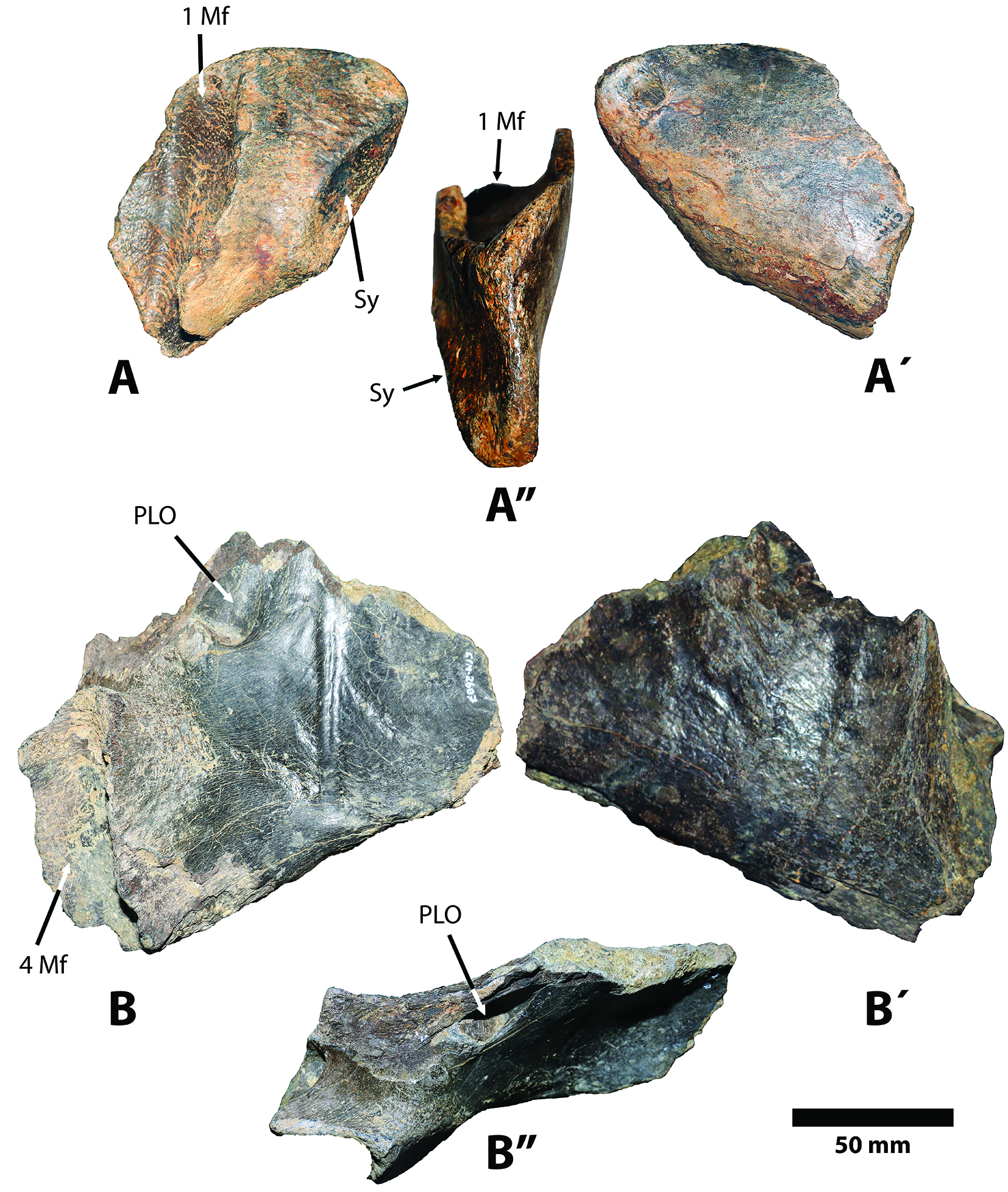
Figure 4 Fragments of the mandible of Sibotherium ka gen. et sp. nov. Anterior part in mesial (A), lateral (A´), and dorsal (A”) views. Posterior part in mesial (B), lateral (B´) and dorsal (B”) views. 1Mf, first molariform; Sy, mandibular symphysis; PLO posterolateral opening of mandible; 4Mf, fourth molariform.
In Sibotherium the distal part of m4, in lateral view, is covered by the anterior margin of the ascending ramus (see Fig. 4.B), as also occurs in Pl. hansmeyeri, Pyramiodontherium, Megatheriops, and several Quaternary Megatheriinae (Brandoni & De Iuliis, 2007[22]; Pujos & Salas, 2004[27]).

Figure 5 Sibotherium ka gen. et sp. nov. Holotype CFM-994. Left astragalus in A, anteromedial; B, fibular; C, dorsomedial; D, posteriomedial; E, ventral; and F, posterior, views. AEF, astragalar ectal facet; ASF, astragalar sustentacular facet; Cub, cuboid facet; DF, discoid facet; FF, fibular facet; Nav, navicular facet; OF, odontoid facet; OP, odontoid process; OT, odontoid tuberosity; ST, sulcus tali.
Astragalus. — The left astragalus of Sibotherium (CFM-994,Fig. 5) has the typical shape of Megatheriinae, with a well-developed and relatively central odontoid process in dorsomedial view, and a navicular facet in anterior view (sensu Brandoni, Carlini, Pujos & Scillato-Yané, 2004)[31].
However, in the Sibotherium astragalus (CFM-994) the odontoid process is not completely centered, and the posterior part of the discoid facet, posterior to the odontoid process, is 50% smaller than the anterior part of that facet anterior to the odontoid process, a condition that is similar to the Megatheriidae from La Venta (Colombia) (UCMP 39593) reported and figured by Hirschfeld (1985), and reported in Megathericulus patagonicus (De Iuliis et al., 2008)[30], but differs from Urumaquia, Megatherium, Eremotherium, Pliomegatherium, Eomegatherium, Pyramiodontherium, where the odontoid process is centered, and the discoid facet posterior and anterior parts to the odontoid process is more or less equivalent in longitude.
Regarding Hirschfeld (1985) the astragalus of Megathericulus patagonicus is smaller and has a less well-developed odontoid process than in the La Venta astragalus (UCMP 39593). The La Venta Megatheriidae astragalus (UCMP 39593) is similar in size to Eomegatherium nanum which is at least 120 mm long (Hirschfeld, 1985). Sibotherium has a well-developed odontoid process larger than that of the La Venta megathere.
In Sibotherium the astragalus is wider than long (CFM-994; length = 153.2mm; wide = 185.6mm; CFM-1654, length =146.7mm; wide =159mm), differing from Urumaquia where the astragalus is longer than wide (length =175; wide =144mm), as in Eremotherium laurillardi (CFM-850 left, length = 265mm; wide = 257mm; CFM-849 right, length = 270; wide = 253; CFM-1871 right, length = 227mm; wide = 224mm) [Figure 6, Figure 7, Figure 8].
In the Sibotherium astragalus (CFM-994), the fibular facet is divided in two main areas. As in other Megatheriinae, an anteroposteriorly elongated dorsal portion joined with the discoid facet dorsally, and a ventral portion extended ventrally and is not in contact with the ectal facet as in many Megatheriinae, except in Megathericulus patagonicus in which the ectal facet and the ventral portion of the fibular facet are in contact (sensu Brandoni, González & Bucher, 2019)[32].
In Sibotherium (CFM-994) the angle between the odontoid and discoid facets in the astragalus is 110º, but in Eremotherium (CFM-1871 collected in Palmares, Costa Rica) it is 115º, and in Urumaquia it is approximately 100º, 90º in Pyramiodontherium bergi and P. scilatoyanei, and between 100º and 120º in Megatherium and Eremotherium laurillardi.
In the left astragalus of Sibotherium (CFM-994) the sulcus tali is short and deep, and in Eremotherium it is as wide as long, and the sulcus tali is longer and shallow.
In Megathericulus patagonicus the navicular facet lies dorsally, with the plane of the discoid facet intersecting it approximately at its middle or two third, as in Megathericulus primaevus Cabrera, 1939; in contrast, it is relatively more ventral in other megatheriines (Brandoni and Carlini, 2009[33]; Brandoni et al., 2019[32]; De Iuliis et al., 2008[30]). The navicular facet is well dorsal to the plane of the discoid facet, that plesiomorphic feature distinguish M. patagonicus, Urumaquia, and Sibotherium from more derived megatheriine where the navicular facet is most ventrally located (De Iuliis et al., 2008[30]; Brandoni, et al., 2019[32]).
The astragalar depression is deep and conical with a very sharp apex. In Sibotherium (CFM-994), Urumaquia and Megathericulus nearly the middle of the navicular facet is dorsal with respect to the plane of the discoid facet, whereas in Pyramiodontherium spp., Eremotherium laurillardi and Megatherium urbinai, only one third is dorsal to this plane (Brandoni and Carlini, 2009)[33]. Carlini, Brandoni & Sánchez (2008a)[34] suggest that the navicular facet in Urumaquia is one third dorsally with respect to the discoid facet, but in their figure 2B it is clear that in Urumaquia the navicular facet is 50% dorsally, as in Megathericulus or Sibotherium.
Sibotherium differs from Urumaquia in having the navicular process and the navicular facet completely to the posterior border of the discoid facet for the tibia, differing from Urumaquia in which both the navicular process and navicular facet are completely anterior to the anterior border of the discoid facet.
The odontoid tuberosity in Sibotherium is not raised as in Megathericulus, or Pyramiodontherium, but is most robust and developed than in Eremotherium or Megatherium, but smaller than in Pyramiodontherium, where it is very robust and highly developed and strongly projected medially raised (sensu Brandoni and Carlini, 2009[33]; De Iuliis et al., 2008[30]).
In Sibotherium the discoid facet and navicular facet are separated by a wide and deep channel more than in Eremotherium eomigrans or E. laurillardi, and the navicular process projects more medially than in any other Megatheriinae, giving the astragalus the appearance of having a neck. The odontoid process and the odontoid facet in Sibotherium are lateral to the navicular facet, differing from many other Megatheriinae (Urumaquia, Megathericulus, Pyramiodontherium, Anisodontherium, Eomegatherium, Eremotherium, and Megatherium) where the odontoid process is posterior to the medial plane of the navicular facet.
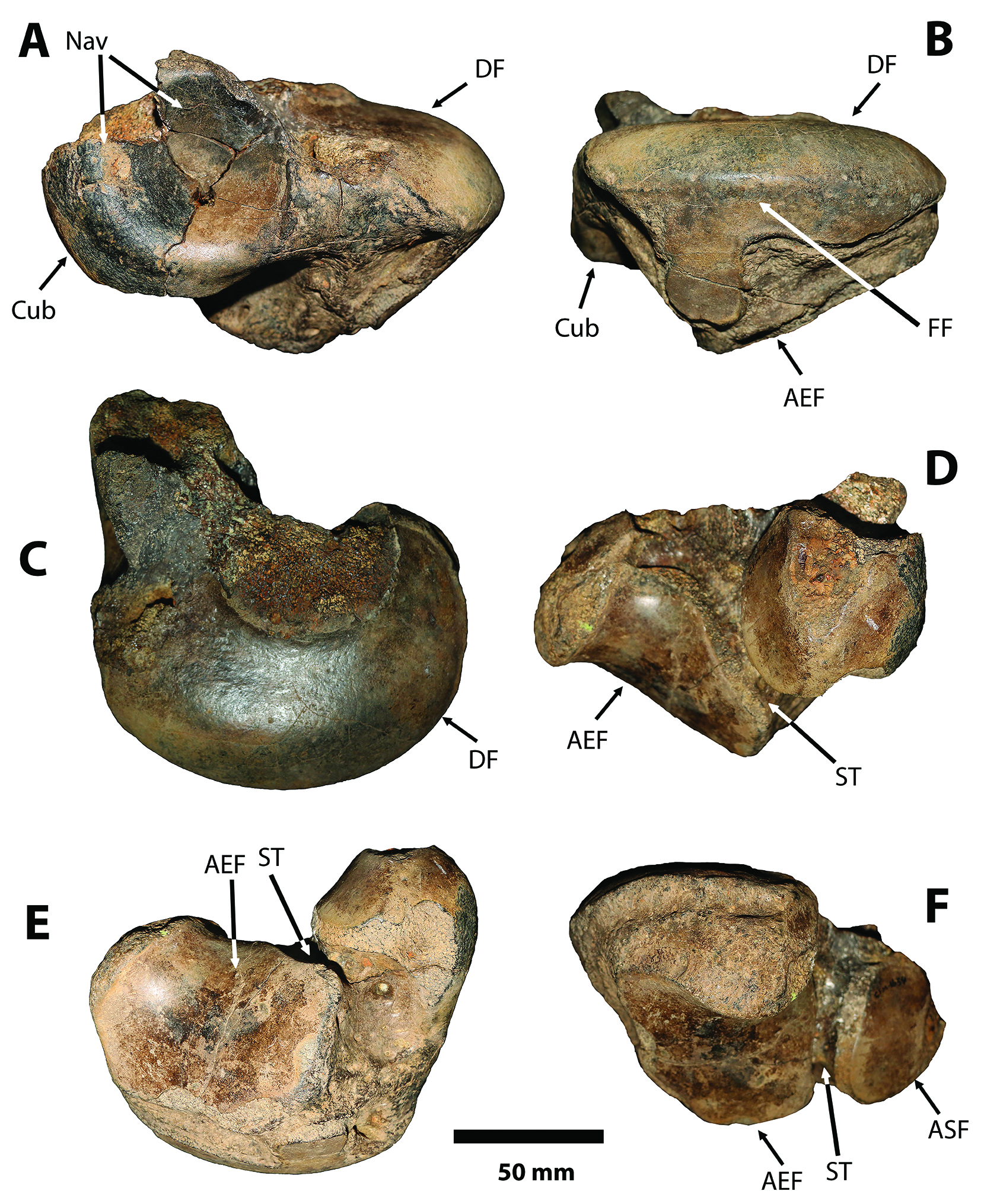
Figure 6 Sibotherium ka gen. et sp. nov. CFM-1654. Left astragalus in A, anteromedial; B, fibular; C, dorsomedial; D, posteriomedial; E, ventral; and F, posterior,views. AEF, astragalar ectal facet; ASF, astragalar sustentacular facet; Cub, cuboid facet; DF, discoid facet; FF, fibular facet; Nav, navicular facet;ST, sulcus tali.
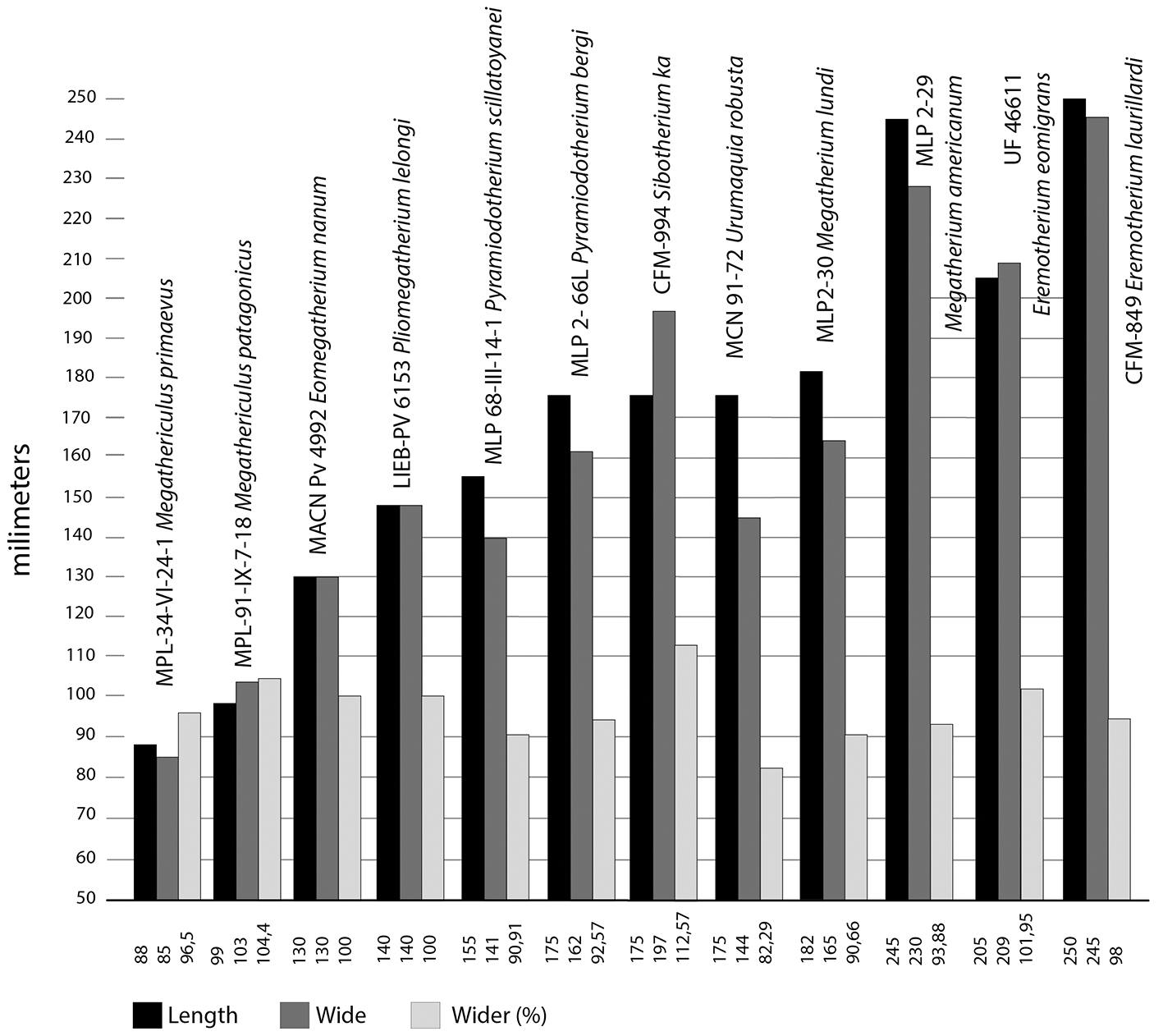
Figure 7 Sibotherium ka gen. et sp. nov. astragalus length by wide graph compare with different Megatheriidae.
In Sibotherium the ventral fibular facet of the astragalus is not connected with the ectal facet as in Anisodontherium, which contrasts with Megathericulus patagonicus where they are connected. (Brandoni & De Iuliis, 2007)[22].
In Sibotherium the sulcus tali in the astragalus is short, narrow and deep and the most anterior part of the sulcus tali is more anteromesial than in Eremotherium where is more anterolateral, longer and shallow.
The sustentacular facet in Sibotherium is short, oval in shape, and its long axis is obliquely positioned with respect to the long axis of the ectal facet, and the sulcus tali is short and also obliquely positioned with respect to the lateral border of the ectal facet. In Eremotherium the sustentacular facet is short and triangular in shape, and its long axis is parallel with respect to the long axis of the ectal facet, and the sulcus tali is parallel with respect to the lateral border of the ectal facet.
CFM-995 corresponds to the right calcaneum without the sustentacular facet and the posterior half of the tuber calcis; CFM-998, left tuber calcis of calcaneum; CFM-1243, fragment of calcaneus just with the ectal facet. The calcaneum is short, robust and widened posteriorly as in Pyramiodontherium scillatoyanei, differing from the other megatheriines where this bone is longer and less robust (De Iuliis, Ré, & Vizcaíno, 2004)[35].
Discusión
Phylogenetic approach
A new Xenarthra taxa is questionable or doubtful when that taxa is erected on single or very few isolated remains, partial or poorly preserve remains, and without consideration that the diagnostic characters fall within the range of known variation in other sloth species (De Iuliis, 2017)[36].
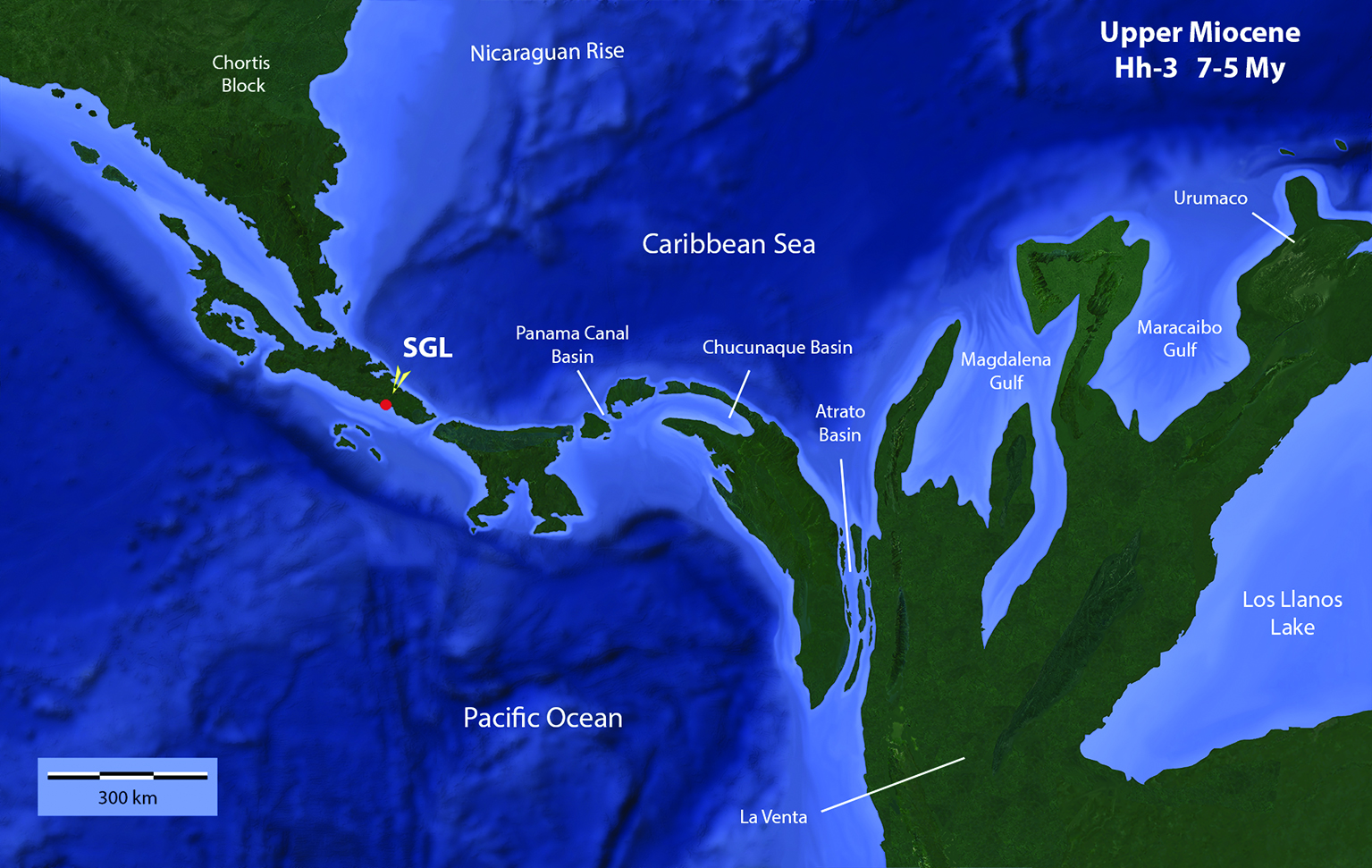
Figure 8 Hypothetical paleogeographical reconstruction of the Chortis Block, Isthmus of Panama and North West of South America at the Upper Miocene. SGL: San Gerardo de Limoncito Locality and site of the found of Sibotherium ka gen. et sp. nov. The Northern portion of Southern Central America probably looked more like a peninsula that allowed the arrival of North American fauna, while the Panamian territory probably looked like an archipelago that when the sea level dropped or a tectonic rising was happening, or both situations coincided, allowed the xenarthrans to overcome the narrow oceanic passages, swimming or rafting. The map was made by one of the authors, Laurito based on the geological maps and other data, published by Albert, Val & Hoorn, 2018[37], Alvarado & Cárdenes, 2016[38] ; Alvarado et al., 2007[39]; Coates, Aubry, Berggren, Collins & Kunk, 2003[40]; Coates, Collins, Aubry & Berggren, 2004[41]; Denyer & Alvarado (2007)[18]; Laurito & Valerio (2012a)[12].
Even though the fossil material is fragmentary, the characters used to diagnose Sibotherium have been used by many authors, including Brandoni & De Iuliis (2007) [22], Carlini et al. (2006, 2018) [10, 26], De Iuliis et al. (2008)[30] and Pujos et al. (2017), among others. Who diagnosed other taxa under similar conditions, from characters that have been interpreted as discrete morphological characters. For example, Brandoni et al. (2019)[32] recognize the importance of the navicular facet depending on the astragalar discoid facet plane; the shape and proportion of the molariforms; the position of the posterior part of the mandibular symphysis respect to the first molariform. All of them, very important discrete morphological characters, used to diagnose basal or advance Megatheriidae, and have been used, depending on their degree of development, their absence or presence, to diagnose taxa. Likewise, De Iuliis et al., 2008[30] propose that the compression or not of the molariforms in Megatheriidae, as well as their relative position respect to the mandibular symphysis and the relative position of the fifth molariform respect to the anterior part of the ascending ramus of the mandible, or even the shape of the lophs on the occlusal surfaces are valid discrete characters to be used to diagnose taxa.
The new Megatheriidae from San Gerardo de Limoncito, Sibotherium ka, is erected on the base of few isolated and partially preserved remain, but we can recognize in those remains many diagnostic features where its unique combination resulting on the distinguishing of a new taxon.
For example, the astragalus of megatheriines is conservative, and few consistent morphologically diagnostic features at the specific level are recognized in this element in Plio- Pleistocene megatheriines (De Iuliis, 1996)[11]. The isolated astragali can rarely be confidently identified to species, because of general morphological similarities (De Iuliis, 1996)[11]. But, apparently for pre Plio-Pleistocene megatheriines (Middle and Late Miocene megatheriines) the astragalus has diagnostic value, and can used to distinguish between taxa. For example, De Iuliis et al. (2008)[30] used some astragalus features, combined with some other cranial and postcranial features in the revised diagnosis proposed for Megathericulus patagonicus, considered the earliest certain megatheriine, dated at 11.8 Ma (Mayoan – Laventan SALMA, Middle Miocene).
However, in Curré Formation 3 different astragali have been found belonging to the same taxon, and associated with cranial and other postcranial elements which allow us to name the new Megatheriinae taxon. It should be clarified that all specimens were recovered within the same outcrop, in an area of not more than 200 square meters, and they keep the same morphology and proportions.
Sibotherium ka shows two plesiomorphic characters of Megatheriinae, the molars mesiodistally compressed (wider than long), and the navicular facet of the astragalus dorsally located with respect to the discoid facet (Brandoni et al., 2019[32]; De Iuliis 1996[11]; De Iuliis et al., 2008[30]; Pujos et al., 2013[2]), which suggests that Sibotherium is a basal member of Megatheriinae as are Megathericulus, Pyramiodontherium, or Anisodontherium.
Probably another plesiomorphic character in Megatheriinae is the developed odontoid tuberosity, which is strongly developed in Miocene Pliocene Megatheriinae (M. patagonicus and Pyramiodontherium bergi), and poorly developed in advanced Megatheriinae (Eremotherium and Megatherium) (Brandoni et al., 2019[32]; De Iuliis et al., 2008[30]). The odontoid tuberosity in Sibotherium is well developed, but not as strongly as in M. patagonicus or P. bergi.
In Sibotherium the posterior end of the mandibular symphysis lies far anterior to the m1, as in Megathericulus patagonicus, Anisodontherium halmyronomum, and Eomegatherium andinum. This is considered by De Iuliis et al. (2008)[30] as another plesiomorphic character opposed to the more posterior position of the symphysis with respect to the m1 in more derived megatheriines (e.g. Eremotherium, Megatherium) (De Iuliis, et al., 2008)[30], but the position of the posterior end of the mandibular symphysis in Pyramiodontherium brevirostrum reaches the plane of the alveolar septum between m1 and m2. That character has been treated as plesiomorphic by De Iuliis (1996)[11].
Pujos et al., (2013)[2] suggested that Megathericulus is a small-sized Megatheriinae (Megatheriinae half the size of Megatheriops or Pyramiodontherium), and Eomegatherium nanum is a middle-sized Megatheriinae. However, De Iuliis et al. (2004)[35] suggest that whereas Pyramiodontherium scillatoyanei is a medium-sized Megatheriinae, with a body mass of approximately 2,500 kg, they considered Pyramiodontherium bergi, Megatherium americanum, Eremotherium laurillardi, and Eremotherium eomigrans as large-sized Megatheriinae. Farina et al. (1998)[42] reported a body mass of Megatherium americanum in a range of 6,073 kg and 2,745 kg. Carlini et al. (2006)[10] suggested that Urumaquia robusta is a large-sized Megatheriinae. The astragalus of Sibotherium ka is similar in size of Pyramiodontherium bergi, Urumaquia robusta, or Megatherium lundi, which allow us to suggest that Sibotherium is a large size Megatheriinae.
Biogeographic and chronological implications
Until now the earliest record of a Megatheriinae in North America, after the formation of the Panamanian Land Bridge, is Eremotherium eomigrans from Haile 7C, latest Blancan (probably between 2.2 and 1.7 Ma), located approximately 6 km NE of Newberry, Alachua County, Florida, USA, and apparently was among the earliest mammalian immigrants to North America (De Iuliis & Cartelle, 1999)[43].
Then San Gerardo de Limoncito ground sloth Sibotherium ka from Costa Rica, represents the first Megatheriidae-Megatheriinae fossil record in Central and North America with a radiometric age (87Sr/86Sr) of 5.80 Ma +/- 0.60/-0.80, and apparently before the closure of the Isthmus of Panama around 2.8 Ma according to the O’Dea et al. (2016)[44] hypothesis.
However, the earliest certain late Tertiary mammalian immigrant in North America from South America is the Megalonychidae Pliometanastes from California and Florida at 8 Ma (Hirschfeld & Webb, 1968[45]; Morgan, 2005[46]), and the Mylodontidae Thinobadistes, known from Florida and Texas, probably with the same age (Webb, 1989)[47]. A southern record of Pliometanastes has been reported from Juchipila, El Resbalón, Mexico with 5.59 Ma (Carranza-Castañeda, Aranda-Gómez, Wang & Iriondo, 2013[48], and from San Gerardo Limoncito (Late Miocene), Costa Rica (Laurito & Valerio, 2012b)[49], with 5.8 Ma old.
The closure of the Panama seaway around 2.8 Ma ago implied that the first dispersal event of Pliometanastes and Thinobadistes, with 8 Ma in North America, must have immigrated by a water route, as island hoppers (Hirschfeld, 1985) or rafting immigrants (Laurito and Valerio, 2012a)[12], with the assumption that ground sloths were durable swimmers and adapted to live in a wide variety of environments (Webb, 1997)[50].
After 2.8 Ma ago the Isthmus of Panama originated as a landmass, so that fully walking mammals and birds (35 families of mammals and one of bird were involved in the GABI) began dispersal across the Isthmus about 2.4 Ma (Webb, 1976, 1997)[50, 51]. Marshall (1988)[52] stated that the first great contingent of South American immigrants appeared in North America near the end of the Blancan at about 2.6 to 2.8 Ma. The “walking” immigrants included two ground sloths, Eremotherium (De Iuliis & Cartelle, 1999)[43] and Glossotherium (Robertson, 1976)[53], armadillos, pampatheres, glyptodonts, capybaras, porcupines and a phorusrhacid bird. The peak of the interchange probably occurred sometime in the Irvingtonian NALMA, but continued into the Rancholabrean.
If we follow the hypothesis of 2.8 Ma for the closure of the Panama seaway, then the ancestor of the Sibotherium ka, from Costa Rica, was dispersed swimming or rafting through the Panama seaway from South America to the ancient Costa Rica terrestrial land mass.
Another hypothesis is that the Panama seaway was closed during middle Miocene times (Montes et al., 2015)[54]. A variation of that hypothesis implies that over the past 10 Ma there has been substantial dispersal and vicariance, maybe associated with landscape formation, volcanism, climate change, and/or sea level fluctuations, across the Isthmus of Panama and that pulses of dispersal of terrestrial organisms occurred over at least three periods during the last 30 Ma (Bacon et al., 2015)[55].
If we follow this hypothesis of an early closure of the Panama seaway and origin of Isthmus of Panama, it implies that the Xenarthra dispersed to North and Central America walking by a terrestrial route, and not swimming or rafting.
The sharks and crocodyles as predators reported in the Curré Formation could represent an ecological barrier for the swimming dispersal route for terrestrial mammals (e. g. Giant Ground Sloths) in the case that Costa Rica and Panama were separate at 5.8 million years old.
There is evidence that some turtle shells and mammalian long bones in the Curré Formation from San Gerardo de Limoncito bone bed deposit, Costa Rica, are broken, some with fractured edges that are slightly abraded, indicating breakage prior to fluvial transport. Numerous bones of turtle, horse, and camel exhibit conical marks, which appear to be punctures and are consistent with those produced by isodont teeth. This suggests that many of the turtles, horses and camel could be the result of prey breakage by crocodilians, which have been reported in that formation (sensu Mead et al., 2006)[56]. This implies that those terrestrial mammals were living near water inhabited by crocodiles.
Also in the conglomeratic facies of the subaquatic Fan Delta of the Curré Formation in San Gerardo de Limoncito Costa Rica has been reported 7 Chondricthyes taxa: Isurus desori, Hemipristis serra, Carcharhinus priscus, Carcharhinus longimanus, Isogomphodon acuarius, Isogomphodon caunellensis, Sphyrna arambourgi (Laurito & Valerio, 2008)[58]. However, there is no evidence of bite marks on terrestrial bones of mammals, but the sharks reported here probably represent another potential predator of terrestrial mammals in the Curré Formation about 5.8 million years ago.
On the other hand, stable isotopes of carbon and oxygen on the enamel fossils of the extinct equids Calippus hondurensis, Dinohippus mexicanus, and Protohippus gidleyi, the gomphothere Gomphotherium hondurensis, and the llama Hemiauchenia vera of the Early–Late Hemphillian (Hh3) from San Gerardo de Limoncito, Puntarenas province, Costa Rica, suggest that these mammals fed mainly on C3 plants and lived in clearings rainforests (Pérez-Crespo et al., 2018)[57].
A probable paleogeographical scenario for migration
One hypothesis considered the possibility that a species morphologically close to Proeremotherium eljebe migrated into North America and gave rise to the early species of Eremotherium (i.e., E. eomigrans from the Blancan, latest Pliocene middle Pleistocene (Carlini et al., 2006, 2008a)[10, 34]. That hypothesis has been supported by the presence of Proeremotherium sp. AMU- CURS 184 from the San Gregorio Formation, Urumaco Venezuela, which seems to be morphologically intermediate between Proeremotherium eljebe and Eremotheriumeomigrans, and it was argued that after the cladogenetic process of Eremotherium giving rise to E. laurillardi in the Rancholabrean (Late Pleistocene) of North America, E. laurillardi migrated to South America and colonized most of the north and central lowlands of South America (Carlini et al., 2008a)[34].
That biogeographic scenario of northern megatheriines, also has proposed for pampatheres (Scillato-Yané et al., 2005)[74], glyptodonts (Carlini et al., 2008b[59]; Carlini and Zurita, 2010[60]; Zurita, Oliveira, Toriño & Krapovickas, 2011[61]) and dasypodids (Castro, Carlini, Sánchez & Sánchez-Villagra, 2014)[62], and assuming a migration from South America during mid/late Pliocene, dispersion and diversification into North America and re-ingression into South America during the latest Pleistocene.
However, Proeremotherium and Eremotherium are evidently close morphologically, and may be phylogenetically, but there is no evidence that Proeremotherium migrated to North America and diversified to Eremotherium and re-entered South America.
Also ,the earliest records of Eremotherium in South America come from El Breal de Orocual, which according to the faunal assemblage probably represent a late Pliocene-early Pleistocene timing (Rincón, Parra, Prevosti, Alberdi & Bell, 2009[63]; Rincón, Prevosti, & Parra, 2011[64]), that means that Eremotherium had been present in Northern South American environment in the late Pliocene/early Pleistocene, in opposition with the Eremotherium late Pleistocene re-entered hypothesis of Carlini et al. (2006, 2008a)[10, 34]. The record of Scirrotherium antelucanus (Pampatheriidae) (Laurito & Valerio, 2013) and Sibotherium ka (Megatheriinae) from San Gerardo de Limoncito, Costa Rica, implies a much earliest presence of the ancestors of these taxa before the closure of Isthmus of Panama 5.8 Ma ago, in the late Miocene, and not in the middle –late Pliocene as Carlini et al. (2018)[26] proposed.
The unknowns posed by the fossil record of mammals at the Upper Miocene of southern Costa Rica, basically are: the dispersion route that these faunas followed; the apparent absence of North American mammals in sediments of similar age in South America (beyond doubt); the apparent northward advanced migration of xenarthrans; and the geological viability of a continuous corridor between the Americas during the late Miocene.
The San Gerardo de Limoncito locality is just over 800 km from the northwestern corner of South America. Its fossil fauna, especially the mammal assemblage, constituted by large beasts and small ones, are indisputable evidence of an exchange between the Americas as early as the Late Miocene (5.8 Ma) and the existence of a terrestrial corridor, if not permanent at least temporary.
The fauna of North American ancestry is represented by the horses Calippus hondurensis, Dinohippus mexicanus and Protohippus gidleyi, the peccaries Prosthennops serus and Protherohyus brachydontus (Laurito & Valerio, 2010[19]; Valerio & Laurito, 2020)[65], the mastodon Gomphotherium hondurensis and the lamini camel Hemiauchenia vera (Valerio & Laurito, 2008[66]; Laurito & Valerio, 2016)[67]. But there must be included within this migratory group Pliometanastes, a Xenarthra derived from a South American ancestor (Morgan, 2008[68], Laurito & Valerio, 2012b)[49], which arrived in North America as early as 8.54 Ma (Hirschfeld, 1981)[69], during the Early Hemphillian (Hh-1) and its fossil record in the late Miocene of Southern Central America could be interpreted as an example of reverse migration.
The South American fauna is represented by the Pampatheriidae Scirrotherium antelucanus Laurito & Valerio, 2013[70] and the Megatheriidae Sibotherium ka. These two species pose two possible migration scenarios: ones in which these animals were able to swim short distances, saving the sea gaps, and a second one, where indeed the land corridor was established for a short period of time either as a product of the tectonic uplift or a drop in sea level or a mixture of both.
Of the two scenarios, the first is the most difficult to demonstrate due to the lack of evidence, apart from the fact that their remains were found in coastal sediments. But the second one is much more likely.
The “widespread of neritic sediments throughout the Darien region during the late Miocene, and the presence of a regional unconformity suggests the completion of the Central American Arc collision with South America by 7.1 Ma”. After that, “no Pliocene deposits are recorded from either the Darien or the Panama Canal Basin, and no sediments younger than 4.8 Ma have been identified in the Atrato Basin of Colombia, suggesting rapid uplift and extensive emergence of the Central American isthmus in the latest Miocene” (Coates et al., 2004)[41]. This evidences a probable partial land corridor between the Northwest of Colombia, the Atrato Basin and the complexly folded and faulted synclinorium that forms the central Chucunaque -Tuira Basin of the Darien (cf. Coates et al., 2004)[41].
On the other hand, a more or less continuous volcanic arc was developed since the Middle Miocene until the Pleistocene (De Boer, Defant, Stewart, & Bellon, 1991[71]; MacMillan, Gans & Alvarado, 2004[72]), which extended from the southern half of Costa Rica to immediately north of the Panama Canal basin.
This scenario allows hypothesizing the existence of long and narrow emerged territories linked to the subcontinents and interrupted by narrow and relatively deep and short interoceanic marine passages, bordered by small islands.
In other words, the presence of North American mammals in southern Costa Rica is sufficient evidence to assume the existence of a continuous land corridor between Southern Central America and the Chortis Block and therefore with North America.
The last element to complete the recipe are the oscillations of the sea level and Zachos, Pagani, Sloan, Thomas & Billups (2001)[73] explain that “a gradual cooling and reestablishment of a major ice-sheet on Antarctica became by 10 Ma and the mean d18O values then continued to rise gently through the late Miocene until the early Pliocene (6 Ma), indicating additional cooling and small-scale ice- sheet expansion on West-Antarctica and in the Arctic”.
Extrapolating, we can assume that the continuous, but not so obvious drop of sea level, along the late Miocene, along with the continued tectonic uprising, driven by the subduction, and the tectonic coupling of the island arc, produced the temporary closure and narrowing of some marine passages at the south of the Panama Canal Basin and northwestern Colombian territories, which allowed the migration of some xenarthrans and possibly additional biota, and this scenery could also explain the later biota exchanges and their episodic pattern, by way of migratory pulses prior to the definitive establishment of the land corridor.
Finally, it is possible that the climatic continuity between the south of Costa Rica, Panama and the Northwest of Colombia, facilitated the migration of South American elements and discouraged the migration of North American mammals adapted to more open forest and dry conditions. This is evidenced from the analysis of the δ13C in the dental enamel from the horses, camels and mastodons associated with Sibotherium ka in SGL, where all of them fed on C3 plants, and it suggests that during the Late-early Hemphillian the site was more humid than today, thus favoring the presence of C3 plants. Together with the δ13C and δ 18O values of the dental enamel in these fossils, the paleovegetation reconstructions made for this geological period suggest that these herbivorous mammals lived in a tropical rainforest, but in clearings of the forest where they fed on plants that developed there (sensu Pérez-Crespo et al., 2018)[57].
Material exanimate to this study
Eremotherium eomigrans astragalus UF 46611, Alachua Co., Haile XVI, Florida, USA.Eremotherium larillardi Astragalus AMNH 95753, San Miguel Lara state, Venezuela, Astragalus CFM-850, San Ramón, Costa Rica. Eremotherium sp. Astragalus OR-1330, El Breal de Orocual, ORS-16, and Astragalus OR-1331, El Breal de Orocual, ORS-16, Monagas state, Venezuela.Urumaquia robusta astragalus MCNC-74-72V (Holotype), Urumaco, Falcon state, Venezuela.














