Introduction
A large number of parasites can be observed in the blood of birds (White et al., 1978). These microscopic organisms have a cosmopolitan distribution, and have been described in several groups of bird hosts in practically every geographic region of the world (Lisbôa et al., 2008). Although there are few records of health changes resulting from trypanosome infection in birds, there is similarity with the manifestation in mammals, and changes in cardiac tissue, liver, and spleen have been observed (Mungomba et al., 1989). In addition, parasitic infections in birds affect the mechanisms of sexual selection, migration, reproduction, competition and, in more severe cases, result in the extinction of certain local bird species (Altizer et al., 2000; McCallum & Dobson, 1995).
Trypanosomes are flagellated protozoa and it is the most abundant and most important genus among kinetoplastids. Trypanosomes are extensively studied because they cause serious diseases of humans (Chagas disease, sleeping sickness) and domestic animals (Taylor et al., 2007). In contrast to their mammalian relatives, avian trypanosomes are in most cases harmless to their hosts and they remain understudied although they are not less interesting (Baker, 1976). The transmission in birds can occur through a variety of vectors, such as mites, lice, culicids, and simuliids (Molyneux, 1977; Svobodová et al., 2017; Votýpka et al., 2012).
Although experimental infections involving vectors and birds have been performed (Bennett, 1961; David & Nair, 1955; Votýpka et al., 2012), aspects of avian trypanosome transmission are poorly understood (Votýpka et al., 2012). However, some of them are known to be transmitted by ingestion of infected vectors or by contamination of the host’s worn skin or conjunctiva with parasites present in the vector’s feces (the hypphoboscid fly Ornithomyia avicularia and the black fly Eusimulium securiforme) (Desser et al., 1975; Mungomba et al., 1989; Svobodová et al., 2017; Votýpka & Svobodová, 2004; Votýpka et al., 2012).
Although some species of the genus Trypanosoma are etiological agents of diseases that represent a public health concern, the vast majority of trypanosomes species remain neglected by research. There is a lack of information about aspects of the biological cycle, hosts and prevalence, morphology, and morphometry data of bird trypanosomes, with the Amazon region being the least studied environment in this respect (Baker, 1976; Valkiūnas et al., 2011).
In recent years, in Brazil and its different biomes, including the Amazon, there has been an increase in studies involving birds and haemosporidians (Anjos et al., 2021; De La Torre et al., 2021; Fecchio et al., 2007; Fecchio et al., 2017; Fecchio et al., 2018; Fecchio et al., 2019; Fecchio, de Faria et al., 2021; Fecchio, Lima et al., 2021; Fecchio et al., 2022; Ferreira-Junior et al., 2017; Ferreira-Junior et al., 2018; Ribeiro et al., 2004; Roos et al., 2015; Sebaio et al., 2012). However, the focus of these studies, although very important, has been on the parasite-host relationships, the life cycles, the vectors and the consequences of these relationships between birds and haemosporidians. Nevertheless, studies on avian trypanosomes remain scarce, especially when focusing on the Amazon region.
In terms of both area and diversity, the Amazon region is the most important habitat for Neotropical birds. About 800 species of birds have been recorded in the Amazonia biome, of which about 265 are endemic to the region (Nores, 2000). The most important families are Psittacidae, Trochilidae, Ramphastidae, Cracidae, Formicariidae, Tyrannidae, Furnariidae, Cotingidae, Pipridae and Dendrocolaptidae (Nores, 2000). Furthermore, the Amazon Basin is highly heterogeneous, with different phytophysiognomies providing a variety of habitats (Silva et al., 2005).
Studies of vertebrate biogeography have shown that the distribution of many species in the Amazon Basin is not continuous, suggesting that similar distribution patterns of these vertebrates are from centers of endemism (Silva & Oren, 1996). Thus, it has been proposed that there are seven recognized areas of endemism in the Amazon, including the Western and Southwestern regions of the State of Pará, in addition to the Belém area of endemism in the capital region of Pará, Brazil (Cracraft, 1985; Silva & Oren, 1996).
In view of the importance of this biome for the diversity of bird species, it is clear that research on hemoparasites is of great importance for the region and can provide relevant information on the different groups of hemoparasites to be studied, as well as the discovery of new parasite species and their vectors. The present study aims to record infection by Trypanosoma sp. in wild birds from a locality in the Brazilian Amazon, in addition to providing information on the infection intensity, prevalence and morphometric characteristics of trypanosomes.
Materials and methods
Location of bird collection: The present study was carried out in the Floresta Nacional do Tapajós (FLONA do Tapajós), a sustainable use Conservation Unit in the Eastern Amazon, in the West of the state of Pará (3°31’1” S & 55°4’23” W), the area has an elevation range of ~55-220 m.a.s.l. The reserve is 527 319 hectares in size, and is delineated to the West by the Tapajós River, to the East by the Santarém-Cuiabá highway (BR-163), to the North by the rural zone of the Belterra municipality and to the South by the Cupari River. In the Tapajós National Forest, there are two forest typologies: i) Floresta Ombrófila Densa Aluvial, which are those located on the banks of the Tapajós River and its tributaries and streams within the Tapajós National Forest; and ii) Floresta Ombrófila Densa de terras baixas, where there is a large occurrence of species of the genus Alchornea, Handroanthus and Ficus, especially Ficus cestrifolia Schott ex Spreng. and the species Tapirira guianensis and Calophyllum brasiliensis (Carvalho et al., 2021).
Bird capture: The birds were captured during June and August 2018 using mist nets 10 m long by 2.5 m high, with a 16 mm mesh. Ten nets were installed in each of the 16 plots sampled, which were 1 km apart and at least 500 meters from the edge of the forest. The nets were set in each plot for two consecutive days during the morning period. The sampling effort was 120 h/net per plot, totaling 1 920 h/net, with inspections carried out at regular intervals of 30 min. The individuals captured were identified to the species level and following the nomenclature and taxonomy established in accordance with the Brazilian Committee of Ornithological Records. The specimens were identified with metal rings provided by the National Center for the Research and Conservation of Wild Birds (CEMAVE), in order to prevent the same bird being sampled more than once.
Blood collection and methods of detection of hemoparasites and morphometry of Trypanossoma sp.: Sterile insulin needles were used to obtain blood samples by puncture of the ulnar (wing) vein. A heparinized microhematocrit capillary tube was then used to collect aliquots of blood. A blood sample was taken from the tube and used to prepare blood smears in triplicate. The smears were air dried. The slides were fixed in methanol and stained with Giemsa stain (RenyLab® Brazil) according to the Romanowsky principle. The morphotypes of trypanosomatidae were evaluated. Light microscopy was used to study hemoparasites. Parasites were photographed in a Zeiss Axioplan light microscope with an Axiocam ERc 5S camera.
Measurements were taken from Trypanosoma sp., including total body length with flagellum (TL), body length along the midline (BL), body width at the center of the nucleus (BW), free flagellum length (F), nucleus length (NL), nucleus width in the central portion (NW), distance from the center of the nucleus to the anterior extremity (NA), distance from the center of the nucleus to the posterior extremity (NP), distance from the center of the kinetoplast to the center of the nucleus (KN), kinetoplast length (KL), kinetoplast width (KW) and distance from the center of the kinetoplast to the posterior end (KP). The Zen Blue Edition 2 software package was used to determine the morphometric characteristics of Trypanosomatidae (Baker, 1956; Baker, 1966; Baker, 1976).
Statistical analysis: To determine the intensity of infection (expressed in parasites/ml), the direct method was used, based on the methodology described by De Carli (2001). This consists of counting all the parasites found in 100 microscopic fields with a magnification of 1 000X, recorded and calculated as follows: 100 microscopic fields is equivalent to 0.2 μl of blood (infection intensity = (N°: number of parasites × 5) × 1 000 = (parasites/ml). The ecological terms used and the infection parameters of prevalence, mean abundance and mean intensity with respectives confidence interval (IC) 95 % were calculated following Bush et al. (1997), using Quantitative Parasitology 3.0 software (Reiczigel & Rózsa, 2005).
To perform the morphometric measurements, due to the low number of trypomastigotes found, we used an N= 10 of trypomastigotes found in the hosts. Thus, the mean values of the morphological regions measured were used to compare the morphometric similarity described for bird trypanosomes using the Bray-Curtis method, with the aid of the Past 4.0 statistical program (Hammer et al., 2001).
Results
A total of 125 birds were captured, all of which belonged to the Passeriformes order, except Veniliornis affinis (Piciformes) (Table 1). The analysis identified the presence of trypomastigotes in three species of Thamnophilidae: Myrmornis torquata (Fig. 1A), Phlegopsis nigromaculata (Fig. 1B) and Willisornis poecilinotus (Fig. 1C); one species of Dendrocolaptidae: Xiphorhynchus guttatus (Fig. 1D) and one species of Conopophagidae: Conopophaga aurita (Fig. 1E). The overall prevalence was 8.8 % (IC= 0.044 to 0.152), mean abundance was 0.22 (IC= 0.10 to 0.48), mean intensity was 2.45 (IC= 1.55 to 4.36) and with an intensity of infection of 27 500 parasites/ml. In this study we identified only one morphotype of Trypanosoma sp. The prevalence per bird family sampled was 7.2 % for Thamnophilidae (n = 55), 2.0 % for Dendrocolaptidae (N = 50) and 50.0 % for Conopophagidae (N = 2).

Fig. 1 Birds species parasitized by Trypanosoma sp. A. Myrmornis torquata, B. Phlegopsis nigromaculata, C. Willisornis poecilinotus, D. Xiphorhynchus guttatus, E. Conopophaga aurita (Scale bar = 2cm).
Table 1 Bird species infected by Trypanosoma sp. and prevalence (per species infected) and the infection intensity (expressed in parasites/mL of blood) in birds from the Tapajós National Forest, Pará, Brazil.
| Hosts | N | Infected Hosts | Infection intensity Parasites / mL | Prevalence |
| Picidae | ||||
| Veniliornis affinis (Swainson, 1821) | 1 | 0 | - | - |
| Thamnophilidae | ||||
| Epinecrophylla leucophthalma (Pelzeln, 1868) | 2 | 0 | - | - |
| Myrmotherula axillaris (Vieillot, 1817) | 1 | 0 | - | - |
| Myrmotherula longipennis Pelzeln, 1868 | 1 | 0 | - | - |
| Myrmotherula menetriesii (d’Orbigny, 1837) | 6 | 0 | - | - |
| Thamnomanes caesius (Temminck, 1820) | 1 | 0 | - | - |
| Thamnophilus schistaceus d’Orbigny, 1835 | 6 | 0 | - | - |
| Sciaphylax hemimelaena (Sclater, 1857) | 1 | 0 | - | - |
| Hypocnemis cantator (Boddaert, 1783) | 1 | 0 | - | - |
| Willisornis poecilinotus (Cabanis, 1847) | 6 | 1 | 20.000 | 0.167 (0.004 - 0.641) |
| Phlegopsis nigromaculata (d’Orbigny & Lafresnaye, 1837) | 17 | 5 | 45.000 | 0.294 (0.123 - 0.544) |
| Rhegmatorhina gymnops Ridgway, 1888 | 5 | 0 | - | - |
| Myrmoborus myotherinus (Spix, 1825) | 1 | 0 | - | - |
| Pyriglena leuconota (Spix, 1824) | 1 | 0 | - | - |
| Myrmornis torquata (Boddaert, 1783) | 6 | 3 | 60.000 | 0.500 (0.153 - 0.846) |
| Conopophagidae | ||||
| Conopophaga aurita (Gmelin, 1789) | 2 | 1 | 5.000 | 0.500 (0.254 - 0.974) |
| Formicariidae | ||||
| Formicarius colma Boddaert, 1783 | 1 | 0 | - | - |
| Dendrocolaptidae | ||||
| Dendrocincla fuliginosa (Vieillot, 1818) | 23 | 0 | - | - |
| Glyphorynchus spirurus (Vieillot, 1819) | 15 | 0 | - | - |
| Dendrocolaptes certhia (Boddaert, 1783) | 1 | 0 | - | - |
| Dendrocolaptes hoffmannsi Hellmayr, 1909 | 2 | 0 | - | - |
| Dendrocolaptes picumnus Lichtenstein, 1820 | 1 | 0 | - | - |
| Hylexetastes perrotii (Lafresnaye, 1844) | 1 | 0 | - | - |
| Xiphorhynchus spixii (Lesson, 1830) | 2 | 0 | - | - |
| Xiphorhynchus guttatus (Lichtenstein, 1820) | 4 | 1 | 5.000 | 0.250 (0.012 - 0.751) |
| Pipridae | ||||
| Manacus manacus (Linnaeus, 1766) | 1 | 0 | - | - |
| Onychorhynchidae | ||||
| Onychorhynchus coronatus (Statius Muller, 1776) | 5 | 0 | - | - |
| Tityridae | ||||
| Schiffornis turdina (Wied, 1831) | 1 | 0 | - | - |
| Platyrinchidae | ||||
| Platyrinchus platyrhynchos (Gmelin, 1788) | 2 | 0 | - | - |
| Passerellidae | ||||
| Arremon taciturnus (Hermann, 1783) | 4 | 0 | - | - |
| Cardinalidae | ||||
| Habia rubica (Vieillot, 1817) | 4 | 0 | - | - |
Values inside the parenthesis represent 95 % confident intervals.
Morphotype characteristics of Trypanosoma sp.
Taxonomic Summary
Hosts: Myrmornis torquata, Phlegopsis nigromaculata, Willisornis poecilinotus, Xiphorhynchus guttatus, Conopophaga aurita.
Type locality: Tapajós National Forest, Municipality of Belterra, Western Pará, Brazil.
Habitat: Blood.
The morphotype of Trypanosoma sp. found (Fig. 2A, Fig. 2B) presents an elongated cell body, predominantly “C” shaped, with two flexures in the anterior region. Granulations are present in the cytoplasm and the maximum width of the body is equal to the maximum width of the nucleus. The nucleus is spherical to ovoid in shape with a karyosome present, an ovoid kinetoplast projecting at the posterior end. The distance between the kinetoplast and the posterior end is evident. An undulating membrane is present and easily recognized, extending along the body with indistinguishable separation from the free flagellum. The flagellum is about one third the size of the cell body (Fig. 2C).
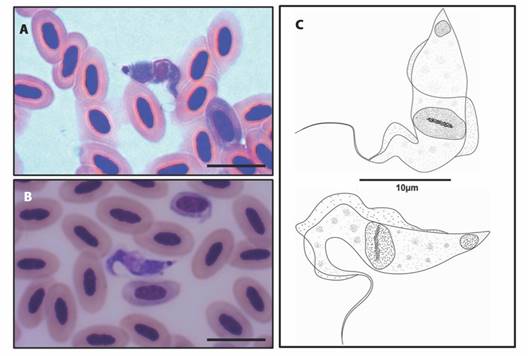
Fig. 2 A. B. Trypomastigote forms of Trypanosoma sp. identified in the peripheral blood of birds from the Brazilian Amazon (10µm bar), C. Schematic drawing of the morphotype of Trypanosoma sp. parasite of five species of birds from the Tapajós National Forest, Brazil.
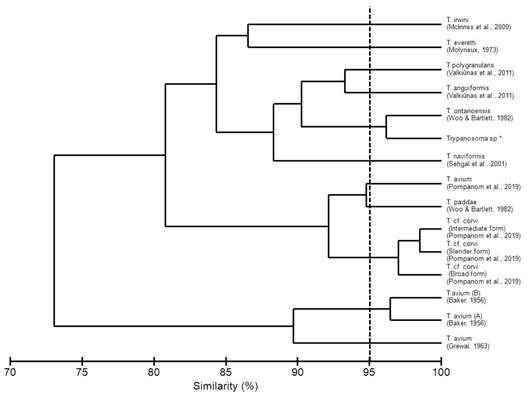
The morphometrics described in the present study were compared with other validated species of avian trypanosomes (Table 2). According to the Bray-Curtis index, a similarity morphometric of 96.15 % with Trypanosoma ontarioensis was indicated (Fig. 3). Despite the high morphological similarity, species determination of the genus Trypanosoma is impossible without the use of molecular methods. This is due to the plasticity of the genus Trypanosoma in other vertebrates, which does not allow species identification based on morphology and morphometry (Spodareva et al., 2018).
Table 2 Morphometric data of trypomastigote forms and of various species of Trypanosoma spp. parasites of birds.
| Measurements (µm) | Trypanosoma sp.* | T. avium (A) (Baker, 1956) | T.avium (B) (Baker, 1956) | T. avium (Grewal, 1963) | T. anguiformis (Valkiūnas et al., 2011) | T. everetti (Molyneux, 1973) | T. polygranularis (Valkiūnas et al., 2011) | T. irwini (McInnes et al., 2009) | T. naviformis (Sehgal et al., 2015) | T. paddae (Woo & Bartlett, 1982) | T. ontarioensis (Woo & Bartlett, 1982) | T. cf. corvi (Slender form) (Pornpanom et al., 2019) | T. cf. corvi (Intermediate form) (Pornpanom et al., 2019) | T. cf. corvi (Broad form) (Pornpanom et al., 2019) | T. avium (Pornpanom et al., 2019) |
| TL | 25.6 | 56 | 51.1 | 43.5 | 32.9 | 22.3 | 30.6 | 40.9 | 22.5 | 42 | 26.4 | 62.8 | 62.4 | 67.1 | 49.4 |
| BL | 17.4 | 52.1 | 43.7 | 40.6 | 28.6 | 17.4 | 20.6 | 30.6 | 22.5 | 36.3 | 18 | 52.4 | 50.7 | 56.3 | 40.5 |
| KP | 0.4 | - | - | 10.7 | 2.3 | 0.9 | 1.8 | 3.6 | 1.9 | 8 | 1.4 | 6.8 | 7.3 | 9.1 | 6.3 |
| NP | 8.9 | 24.9 | 20 | 19.5 | 16.2 | - | 13.3 | 0 | 11.2 | 19 | 8.3 | 21 | 20.8 | 24.5 | 17.2 |
| NA | 8.5 | - | - | - | 11.8 | 7.7 | 13.3 | 11.9 | 11.1 | 17.3 | 9.6 | 27.2 | 29.2 | 30.3 | 20.8 |
| KN | 8.4 | - | - | - | 14.1 | 7.9 | 11.6 | 10.3 | 9.2 | 11 | 6.6 | 12.6 | 13.1 | 13.6 | 9.7 |
| FF | 8.3 | 6.2 | 7.4 | 7 | 4.3 | 7 | 10 | 10.3 | 0 | 5.7 | 8.4 | 10.4 | 11.7 | 10.8 | 8.9 |
| BW | 4.4 | 4.6 | 4.6 | 5.2 | 2 | 5.2 | 3.5 | 3 | 4.4 | 3.1 | 2.6 | 3.8 | 5.5 | 7.2 | 6.4 |
*Present study. The references below each Trypanosoma species indicate where measurements were taken to compare. Morphological location of the measured regions of Trypanosoma spp.: Total body length with flagellum (TL), body length along the midline (BL), body width at the center of the nucleus (BW), free flagellum length (FF), distance from the center of the nucleus to the anterior extremity (NA), distance from the center of the nucleus to the posterior extremity (NP), distance from the center of the kinetoplast to the center of the nucleus (KN) and distance from the center of the kinetoplast to the posterior end (KP).
Discussion
Various biological and ecological characteristics of the host have been associated with the prevalence of hemoparasites in birds. In general, studies on the effects of avian hemoparasites on the biological and ecological characteristics of birds have focused on characteristics such as sex, age, body mass, geographic area, nest type, foraging layers, and feeding habits. Little is known about the relationship between feeding habits and hemoparasite susceptibility, although all positive birds in our study were insectivorous.
Previous studies on blood-borne parasites in birds have shown that species of birds that belong to insectivorous groups are more susceptible to infection with Haemoproteus and Plasmodium (Laurence et al., 2013). Thus, hemoparasites of the genus Plasmodium sp. the causative agent of avian malaria, have been described as a potential cause of extinction and population decline in many bird species, reducing host fitness and in some cases causing death (Cannell et al., 2013, Paxton et al., 2016). Notably, there are no studies on the prevalence aspects of infection with these associations for the avian trypanosome.
The differential susceptibility of bird groups, in relation to hematozoans, may derive from differences in the reproductive biology of different species and habitat type, as well as factors such as small sample sizes (for some species or families) and sampling in different seasons of the year (Norris et al., 1994). In addition, infection may depend on biological parameters such as sex, age, plumage color and body condition, according to (Norris et al., 1994).
Bird trypanosomes have been described in approximately 100 species based on morphological features and host-specific factors, but only a few morphological features have been sufficiently described, because of inadequate illustrations and descriptions (Valkiūnas et al., 2011). Although the morphometry of the parasite may vary during the life cycle, its morphological characteristics (body shape, free flagellum, undulating membrane, kinetoplast position and morphology) are well conserved (Valkiūnas et al., 2011). We found the same relationship when we performed similarity tests with T. anguiformis and T. polygranularis that had been characterized both morphologically and molecularly by Valkiūnas et al. (2011).
Morphometrically, similarities were evidenced when we compared the morphotype analyzed in the present study with T. ontariensis. There was a similarity in the size of the cell body, the distance from the posterior region to the center of the nucleus, the distance from the center of the nucleus to the anterior end, and in the length of the free flagellum, with these characteristics differentiating the morphological types of T. avium, T. anguiformis, T. polygranularis, T. irwini, T. naviformis, T. paddae and T. corvi. Despite the close morphometric similarity, it is not possible to consider identification at the species level only using the morphometric technique, due to the notable plasticity of Trypanosoma spp. (Pornpanom et al., 2019; Spodareva et al., 2018; Valkiūnas et al., 2003).
The present study corroborates the findings of Woo and Bartlett (1982), in which there is no parasitic specificity for T. ontariensis, with infection being observed in five different species of hosts. According to Woo and Bartlett (1982), T. ontariensis biologically resembles T. corvi and T. avium, and can be transmitted experimentally to a wide variety of birds, with low parasitemia being observed in all experimental infections.
The morphometry in the present study was significant when compared to that of T. avium and T. corvi. According to (Votýpka et al., 2004; Votýpka et al., 2012) the species T. corvi and T. avium belong to the “avium” group where the large straited trypanosomes of birds are grouped. These morphological types present similar forms, described in different hosts in the New and Old Worlds. Studies carried out by (Mungomba et al., 1989) stated that the representatives of the “avium” group suggest a complex group of species, with robustness in the cell body and free flagellum, findings corroborated by the comparison with the morphotype found in the present study.
Experimental studies of the trypanosome life cycle of birds show that many Trypanosoma species can be easily distinguished using morphometry alone during co-infections (Molyneux, 1977; Mungomba et al., 1989). In relation to the variability of morphological forms (particularly during development in vectors and in the initial morphogenesis of the metacyclic forms in birds), the hematozoic trypomastigote form is relatively fully developed, with a morphology with highly conserved characteristics, i.e. body shape, body size limits, kinetoplast position and morphology, characteristics of the flagellum and undulating membrane, and morphometric índices (Baker, 1956; Bennett, 2008; Chatterjee & Ray, 1971; Molyneux, 1977; Votýpka et al., 2004).
The diversity of trypanosome species in Amazonian birds is still poorly known. This may be related to logistical difficulties as well as to technical and structural limitations (molecular biology laboratories) in the field. In our study, we provided data on the occurrence of Trypanosoma sp. and target species, as well as morphological and morphometric data of one morphotype, which could be used in more robust studies using integrative techniques of morphology, morphometry and molecular biology methods. Even though the diversity of trypanosome species is not known, it is suggested that studies involving hematophagous invertebrates be designed to better elucidate aspects of the parasite-host relationship in Amazonian birds.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgments section. A signed document has been filed in the journal archives.












 uBio
uBio