Introduction
Different uses of the land have caused changes in the landscapes, promoting strong environmental impacts on the biota (Benítez-Malvido et al., 2022). In this context, the process of urbanization and rise of agricultural frontiers are responsible for the fragmentation of habitats and promote changes in the dynamics and structure of forests along the urban-rural gradient (Overdyck & Clarkson, 2012; Pivello et al., 2006; Santos et al., 2020). For example, the fragmentation will lead to reduced species richness and changes in floristic composition of aboveground vegetation. These changes promote a significant reduction in the ecosystem services of habitat and resource to wildlife, and microclimate for the establishment and growth of plants. Consequently, there may be a significant reduction in the pollination and dispersion of diasporas processes (Benítez-Malvido et al., 2022; Overdyck & Clarkson, 2012) and, therefore, causing changes in some parameters of seed rain, such as density, richness, species composition, habit, and successional category of plants (Overdyck & Clarkson, 2012; Pivello et al., 2006). Thus, the disturbance degree reaching an environment may influence seed rain, and hence the process of natural regeneration (Benítez-Malvido et al., 2022; César et al., 2017; Pivello et al., 2006).
Seed rain represents one of the most important mechanisms in the forest's regeneration process, enabling the entry of seeds and consequently establishment and recruitment of new individuals and species in the forest fragments (Jara-Guerrero et al., 2020; Werden et al., 2021; Yang et al., 2020). The compilation of works carried out in tropical rain forests shows that the urban fragments are surrounded by residences, industries, roads, and highways, while rural fragments are surrounded by sugarcane and Eucalyptus spp. monocultures, pastures, and greenery; these differences in the surrounding matrix of fragments cause changes in the attributes of seed rain (Freitas et al., 2013; Overdyck & Clarkson, 2012). For example, species richness and seed density from the seed rain are higher in rural fragments than in urban fragments (Campos et al., 2009; Cole et al., 2010; Cutway & Ehrenfeld, 2010; Freitas et al., 2013; Knorr & Gottsberg, 2012); species richness and seed density of the seed rain may be higher during the dry season or dry-rainy transition in urban and rural fragments (Gratzer et al., 2022; Jara-Guerrero et al., 2020); seed rain composition differs between urban and rural fragments (Overdyck & Clarkson, 2012).
The predominant factors and practices in each type of surrounding matrix (i.e., sugarcane monoculture and urban centers) may affect the dynamics of the forests and interfere in the seed characteristics of each fragment. Then, it is expected that surrounding type of the rural fragment plays an important role in the species richness of the seed rain and affecting the floristic composition. The surrounding matrix of rural fragment is more uniform. Consequently, there is a greater flow of species in fragments with an agricultural surrounding when compared to the fragments with urban surrounding (Knapp et al., 2008; Lososová et al., 2006). We also need to consider the existence of other adjacent fragments to the rural studied, as they may influence the seed entry in the area, depending on species dispersal syndrome. In this way, we could not discard the possibility of seed arrival from other fragments near the rural, mainly because the composition of the seed rain from a certain area is closely related to its surroundings, i.e., with the immediate neighborhood and with the landscape in which it is inserted (Gratzer et al., 2022; Werden et al., 2021). Therefore, it is expected that the type of surroundings leads to changes in species richness, seed density, floristic composition, and functional attributes of seed rain.
It was also observed that in the rural or urban fragments there is a predominance of trees species, zoocoric dispersal syndrome, and pioneer species (Cole et al., 2010; Freitas et al., 2013; Marangon et al., 2010; Oliveira et al., 2011). However, the proportion of the habit, dispersal syndrome, and successional category of seed rain varies according to the fragments type (rural or urban) and seasons.
Few studies have incorporated both the spatial context and temporal variability in the analysis of seed rain abundance, composition, and taxonomic and functional diversity. Therefore, our study aims to characterize and compare species richness, seed density, floristic composition, habit, dispersal syndrome, and successional category of seed rain in urban and rural fragments of Atlantic Forest, between climatic seasons and years. Considering that characteristics of seed rain in fragments of tropical rain forests may vary depending on the type of environment, climatic seasons, and years, we assume the hypotheses: 1) The species richness and seed density will be highest in the rural fragment during the rainy and dry seasons and during the years; 2) the floristic composition of seed rain differs between fragments, and this difference continue along years and seasons; 3) the variations in the functional attributes of dispersal syndrome (Van Der Pijl, 1982), habit, and successional category (Gandolfi et al., 1995) are explained by the surrounding matrix of rural and urban fragments, and seasonal and annual variation in precipitation.
Study area: The study was carried out in two remnants of Atlantic rainforest in Northeastern Brazil, which have been protected as integral protection Conservation Units (C.U.) since 1987. They are part of a complex of protected areas, inserted in the Metropolitan Recife Region (CPRH, 2003) (Fig. 1). The C.U.s boundaries were mapped on a mosaic of orthophoto maps at a scale of 1: 20 000, dated from the 1980s.
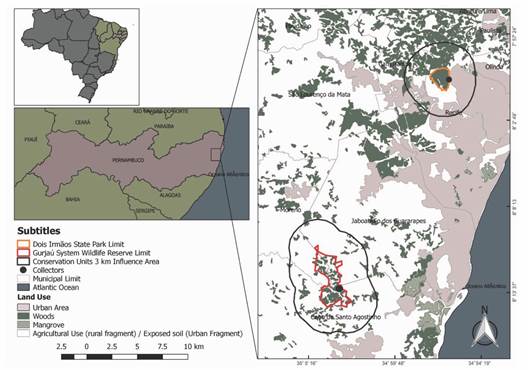
Fig. 1 Location of the urban and rural fragments of Atlantic rainforest evaluated in the metropolitan region of Recife.
The urbanization degree on the surroundings of forest remnants was defined by mapping and quantification of urban and rural occupations in the area that extends from the forest boundary up to 3 km (buffer), according to the methodology presented by Guerra (2016). The mapping was based on the Atlas of the Atlantic Forest Remnants, in partnership with the National Institute of Space Research (INPE). For the Atlas work, were used OLI images of the Landsat 8 satellite and the Google Earth program, from the period of 2009 to 2015 and updated mainly in the second half of 2016. These images are of high spatial resolution and scale approximately of 1:50 000. In the rural and urban fragments, field conferences were also held to verify the presence of consistent changes in the use of the surrounding soil within the mapped area.
Urban fragment was considered that with residential, commercial, or industrial agglomerates, roads, highways, and areas without vegetation, with exposed soils in the vicinity of these agglomerates; the rural fragment is that predominates agro-pastoral activities, small villages and roads, highways, and areas without vegetation distant from urban centers (Guerra, 2016). The urban fragment (08°01'15.1'' S & 34°56'3.2'' W) is characterized by a single vegetation stretch in different stages of secondary succession; and has land use in its surroundings sites, farms, villages, roads, and highways. (Santos et al., 2020). The rural fragment (08º10'00'' & 08º15'00'' S, 35º02'30'' & 35º05'00'' W) in its surroundings have predominantly sugarcane production, and some areas of subsistence agriculture, fruit growing, livestock, and timber extraction (Lyra-Neves et al., 2004; Santos et al., 2020). Mapping of the buffer area of urban fragment recorded a total area of 5 540 ha, whose 30 % corresponds to the native forest area, 33 % the urban area, and 37 % were not defined by the mapping/sensor. Mapping of the buffer area of rural fragment recorded a total area of 10 349 ha, whose 41 % corresponds to native forest area, and 59 % to areas of agricultural use (Fig. 1).
A total of 66 species are presents in the urban fragment and 52 in the rural. The family with the greatest species richness in the urban and rural fragments is Euphorbiaceae. Twenty-six species are present only in the urban fragment; and 12 in the rural fragment; and 40 are commons in both fragments. The floristic similarity by Sorensen index is 68 % (Santos et al., 2020).
The fragments were chosen because they have common characteristics: the same As' climate type (hot and humid) (Kottek et al., 2006), the same soil type (latosols) (Embrapa, 1998; Jacomine et al., 1972), the same vegetal formation (Lowland Ombrophylous Forest) (IBGE, 2012), and average annual rainfall with little variation (2 450 - 2 460 mm). Rainfall totals in rural and urban fragments were, respectively, 1 666.11 and 1 973.8 mm in 2015, and 1 445.50 and 1 602.00 mm in 2016.
Seed rain sampling: For the sampling of seed rain in each fragment (urban and rural), 36 collectors were installed from 5 m of the border, totaling 72. The collectors were distributed in three transects of 300 m, approximately interspaced 100 m away. In each transect, 12 equidistant collectors were installed at 25 m. Our collectors covered a total area of 6 ha (border-center direction). The collectors are 0.25 m2 area (0.5 m × 0.5 m), and they were installed 30 cm above the ground. This method is appropriate to include herbaceous plants (Benítez-Malvido et al., 2022; Knorr & Gottsberger, 2012). These were made in a square shape, with 1 mm nylon mesh, and a depth of ≈ 20 cm, whose function is to retain all the deposited material (César et al., 2017), and they were fixed in tree trunks with nylon threads and identified with a numbered plaque. The material was collected monthly (Freitas et al., 2013) during two consecutive years (February 2015 to January 2017). Each collected sample was packed in polyethylene bags, labeled according to the collector numbering, and placed to dry in stove with a controlled temperature of 65 °C.
At the laboratory, the seeds were manually separated from other materials eventually found, quantified, and classified with the aid of a stereomicroscope. The fruits present in the samples were opened for the seed's removal. Seeds were separated, classified by morphotype, and numbered, until their taxonomic identification according to Angiosperm Phylogeny Group System (The Angiosperm Phylogeny Group et al., 2016). The spelling of species names was verified at the Index Kewensis (www. ipni.org/ipni/plantnamesearchpage.do) and the Missouri Botanical Garden's (MOBOT) database (www.mobot.mobot.org/W3T /Search/vast.html). Abbreviation of the author names of the species was made by consultation with Brummit and Powell (1992) and MOBOT. The number of seeds was expressed as seed/0.25 m2. For seed identification, we used the specific bibliography (Barroso et al., 1999; Lorenzi, 1992; Lorenzi, 1998a; Lorenzi, 1998b; Lorenzi, 2009), besides taxonomy specialist help. In addition, a survey of the individuals close to the collectors was carried out, mainly of the individuals with the reproductive material in the collection period.
Collected seeds were classified according to the Van Der Pijl (1982) proposal in anemochoric, zoochoric, and autochoric. The species were classified as tree, shrub, woody climbing plant (lianas), and herbaceous and in relation to the successional category in pioneers, early secondary, and late secondary (Gandolfi et al., 1995). The syndrome and the successional category corresponding to each species was evaluated by consultations in the local literature (Marangon et al., 2010; Oliveira et al., 2011; Silva & Rodal, 2009).
Data analysis: Differences in the species richness of seed rain between the rural and urban fragments over time were verified by the rarefaction analysis using Estimate S 9.1.0 (Colwell, 2013). The sample-based rarefaction curve shows the mean species number (± standard deviation). Plotted values are means of 100 randomizations. There is significant difference when deviations do not touch (Colwell, 2013; Gotelli & Colwell, 2001).
The Generalized Linear Model (GLM) was used to verify the effect of predictor variables (urban and rural fragment, seasonal and annual variation) on seed density. The differences in mean seed density between urban and rural fragments, during dry and rainy seasons, and during 2015 and 2016 years were verified by the Tukey a posteriori test. These analyzes were carried out by the Statistic software 7.0 (StatSoft Inc., 2003).
The floristic composition of the seed rain between the rural and urban fragments, and in each fragment between seasons and between years was compared through the Non-Metric Multidimensional Scaling (NMDS) Analysis, using the Bray-Curtis dissimilarity matrix, based on the species density present in the 72 collectors from the study areas. ANOSIM was used to verify the significance of the formed cluster in the NMDS. To verify the contribution of the density of each species between rural and urban fragments and in each fragment between years we performed the SIMPER analysis (Similarity percentage). NMDS, ANOSIM, and SIMPER analyzes were performed at the Primer version 7 software (Clarke & Gorley, 2015).
Differences in species abundance among the fragments, years, and seasons within the functional attributes habit, dispersal syndrome, and successional category were verified by Log-Linear model (Sokal & Rohlf, 1995). Only models with significant interactions were considered. The model significance was verified by the Chi-Square test. Analyzes were performed in the Systat Software 10.0 (Systat Software Inc, 2006).
Results
Species richness: During the two years of study, a total of 99 species were found in the seed rain, being 80 species in the urban fragment and 61 in the rural (SMT1). From the total number of species, eight were identified at the family level, four at genus, 69 at the specific level, and 17 morphospecies. Additionally, 39 species occurred exclusively in the urban fragment and 19 in the rural fragment (SMT1).
The families with the greatest species richness in the urban fragment during the two years study were Euphorbiaceae (with four species), Annonaceae, Chrysobalanaceae, Erythroxylaceae, Lauraceae, Malpighiaceae, Melastomataceae, Moraceae, Rubiaceae, and Sapindaceae (with three species each); and in the rural fragment were Fabaceae (with eight species), Anacardiaceae, Annonaceae, Erythroxylaceae, Euphorbiaceae, Melastomataceae, Moraceae, and Sapindaceae (with three species each).
The total species richness of seed rain from the urban fragment was significantly higher than the rural (Fig. 2A). The richness was significantly higher in the urban fragment in 2015 (Fig. 2B) and 2016 (Fig. 2C). Considering each season separately, during the rainy season the species richness was significantly higher in the urban fragment (Fig. 2D), while in the dry season it was similar among the fragments (Fig. 2E).
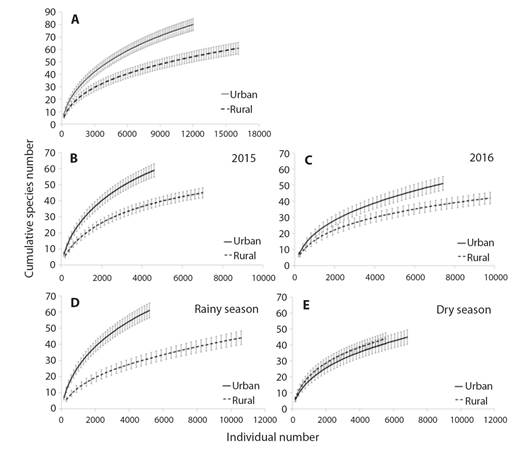
Fig. 2 A. Sample-based rarefaction curve with the mean species number (± standard deviation) from the seed rain in rural and urban fragments; B. in 2015; C. in 2016; D. in rainy and E. dry season; of Atlantic Forest in Northeast Brazil. There is significant difference when deviations do not touch (Colwell, 2013; Gotelli & Colwell, 2001).
Seeds density: The GLM analysis showed that there was a significant effect of fragment, climatic season, and their interaction on seed density of seed rain (Table 1). The Tukey test showed that the mean seed density (± 95 % CI) of rural fragment was higher than the mean seed density (± 95 % CI) of urban fragment in 2016 (F(1, 1720) = 0.59602; P = 0.046437; Fig. 3A) and during the rainy season (F(1, 1720) = 11.144; P = 0.000067; Fig. 3B). There was no significant difference in mean seed density (± 95 % CI) between the rural and urban fragments in 2015 (F(1, 1720) = 0.59602; P = 0.434458; Fig. 3A) and during the dry season (F(1, 1720) = 11.144; P = 0.989715; Fig. 3B).
Table 1 General Linear Model (GLM) analysis showing the influence of the rural and urban fragments, climatic season, years, and their interactions on the seed density of seed rain in an Atlantic Forest in Northeast Brazil
| DF | SS | MS | F | P | |
| Intercept | 1 | 2 723.697 | 2 723.697 | 1 246.575 | 0.000000 |
| Year | 1 | 3.044 | 3.044 | 1.393 | 0.238052 |
| Fragment | 1 | 18.380 | 18.380 | 8.412 | 0.003775 |
| Season | 1 | 8.859 | 8.859 | 4.055 | 0.044202 |
| Year*Fragment | 1 | 1.302 | 1.302 | 0.596 | 0.440208 |
| Year*Season | 1 | 3.179 | 3.179 | 1.455 | 0.227893 |
| Fragment*Season | 1 | 24.349 | 24.349 | 11.144 | 0.000861 |
| Year*Fragment*Season | 1 | 0.321 | 0.321 | 0.147 | 0.701640 |
| Error | 1720 | 3 758.105 | 2.185 | ||
| Total | 1727 | 3 817.539 |
P value < 0.05 denote significant difference (DF = degrees of freedom, SS = sun of square, MS = mean square, F = Fisher test, P = significance).
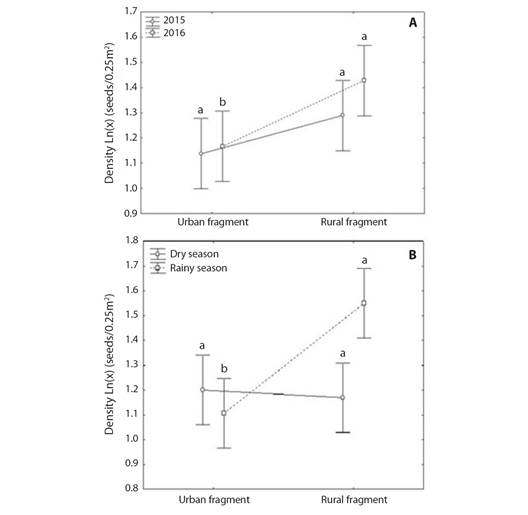
Fig. 3 A. Mean seed density variation in urban and rural fragments of Atlantic Forest in Pernambuco, during 2015 and 2016 years and B. during dry and rainy seasons. Different lowercase letters denote significant difference between the urban and rural fragments by the Tukey HSD test at 5 %. Vertical bars denote 95 % of the confidence interval.
Species composition: The ANOSIM analyze showed that the seed rain composition of the rural fragment was different from the urban fragment (Rglobal = 0.317; P = 0.001). The formed cluster in the NMDS can be seen in Fig. 4A. The floristic composition among the fragments was different in 2015 (Rglobal = 0.226; P = 0.001; Fig. 4B) and 2016 (Rglobal = 0.254; P = 0.001; Fig. 4C). The floristic composition between the fragments was different in the rainy season (Rglobal = 0.225; P = 0.001; Fig. 4D) and in the dry season (Rglobal = 0.295; P = 0.001; Fig. 4E).
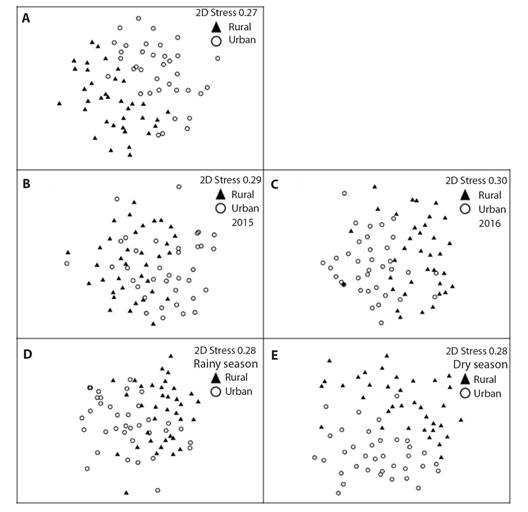
Fig. 4 A. Non-Metric Multidimensional Scaling (NMDS) analysis of seed rain species between rural and urban fragments of the Atlantic Forest in Pernambuco, Brazil; and B. in the 2015 and C. 2016 years; and D. in the rainy and E. dry season, based on species richness. Graph symbols represent the species present in seed rain of each fragment.
SIMPER analysis showed high dissimilarity in the seed rain between the rural and urban fragments (80.11 %), and this behavior remained over the years in each fragment (Table 2). The five species that most contributed to the differences between the fragments were Miconia amacurensis Wurdack, Miconia prasina (Sw.) DC., Maprounea guianensis Aubl., Schefflera morototoni (Aubl.) Manguire, Steyerm. & Frodi, and Cecropia pachystachya Trécul., with a cumulative percentage of 31.4 %. In rural areas, the species that contributed most to the differences were Miconia prasina, Maprounea guianensis, Schefflera morototoni, Cecropia pachystachya, Helicostilis tomentosa (Poepp. & Endl.) Rusby, and Miconia minutiflora (Blonpl.) DC., with an accumulated percentage of 38.83 %. In the urban area, the species that contributed most to the dissimilarities were Miconia amacurensis, Schefflera morototoni, Asteraceae sp1, Cecropia pachystachya, and Parkia pendula (Willd.) Benth. ex. Walper., with an accumulated value of 31.81 %.
Table 2 SIMPER analysis between rural (R) and urban (U) fragments between years (2015 and 2016) and in each fragment, including the species contribution in the dissimilarity between the sampled fragments
| R and U | R between years | U between years | |||
| D.L. = 80.11 | D.L.= 77.55 | D.L. = 79.43 | |||
| Species | D.L. | Species | D.L. | Species | D.L. |
| Miconia amacurensis | 7.68 | M. prasina | 9.38 | M. amacurensis | 9.89 |
| Miconia prasina | 6.89 | M. guianensis | 8.92 | S. morototoni | 5.87 |
| Maprounea guianensis | 5.71 | S. morototoni | 8.38 | Asteraceae sp1 | 5.85 |
| Schefflera morototoni | 5.57 | C. pachystachya | 7.69 | C. pachystachya | 5.13 |
| Cecropia pachystachya | 5.55 | H. tomentosa | 4.46 | P. pendula | 5.07 |
| Asteraceae sp1 | 4.22 | M. minutiflora | 3.92 | G. blanchetiana | 4.37 |
| Parkia pendula | 4.11 | P. pendula | 3.81 | S. salzmanniana | 4.13 |
| Gouania blanchetiana | 3.57 | G. blanchetiana | 3.60 | T. guianensis | 3.72 |
| Helicostilis tomentosa | 3.24 | Asteraceae sp1 | 3.54 | M. prasina | 3.63 |
| Miconia minutiflora | 2.84 | A. dolichocarpa | 2.66 | H. tomentosa | 3.07 |
| Serjania salzmanniana | 2.83 | M. amacurensis | 2.48 | A. dolichocarpa | 3.07 |
| Anaxagorea dolichocarpa | 2.83 | B. adenopoda | 1.91 | L. kunthiana | 2.41 |
| Tapirira guianensis | 2.60 | O. glomerata | 1.54 | O. glomerata | 1.95 |
| Ocotea glomerata | 1.61 | P. heptaphyllum | 1.53 | P. schomburgkiana | 1.76 |
| Licania kunthiana | 1.36 | T. guianensis | 1.03 | P. heptaphyllum | 1.34 |
| Protium heptaphyllum | 1.23 | T. spruceanum | 0.85 | Morfo 29 | 1.21 |
| Banisteriopsis adenopoda | 1.21 | Passiflora sp1 | 0.85 | Passiflora sp1 | 0.98 |
| Pogonophoschomburgkiana | 1.21 | D. guianensis | 0.74 | T. brevistaminea | 0.95 |
| Passiflora sp1 | 1.06 | X. frutescens | 0.67 | E. citrifolium | 0.86 |
| Tovomita brevistaminea | 0.80 | M. aegyptia | 0.66 | B. adenopoda | 0.81 |
| Morfo 29 | 0.76 | P. schomburgkiana | 0.66 | C. mollis | 0.76 |
| Erythroxylum citrifolium | 0.67 | S. salzmanniana | 0.64 | S. rugosum | 0.64 |
| Thyrsodium spruceanum | 0.52 | E. ovata | 0.49 | S. densiflorum | 0.57 |
| Xylopia frutescens | 0.50 | S. hilarii | 0.57 | ||
| Serjania hebecarpa | 0.49 | S. hebicarpa | 0.75 | ||
| Merremia aegytia | 0.49 | B. lucida | 0.55 | ||
| Coccoloba mollis | 0.45 | P. violaceus | 0.48 | ||
| Eschweilera ovata | 0.41 | G. americana | 0.43 | ||
| Simarouba amara | 0.39 | B. virgiloides | 0.41 | ||
| Sloanea guianensis | 0.34 | M. salzmannii | 0.41 | ||
| Pterocarpus violaceus | 0.32 | E. ovata | 0.39 | ||
| Soroceae hilarii | 0.31 |
(D.L = Dissimilarity Level).
Functional attributes: The log-linear analysis for the abundance of seed rain of the studied species showed a significant second-order interaction among the variables fragment, season, and habit (c2 = 9.20; df = 3; P = 0.0268). This means that seed abundance is not explained in isolation by any of these variables. Then, they should be evaluated together. Although in the general set, the total density of tree seeds was higher than the seed density of the other habits, it was observed that the proportion of seeds of trees, shrubs, herbaceous, and woody climbing plant (lianas) changes according to the seasons and the fragments. For example, the seed number of woody climbing plant species was higher in the dry season than in the rainy season. However, in the urban fragment, the variation between dry and rainy season was higher (4.53 times more seeds in the dry season) than the rural fragment (1.73 times more seeds in the dry season) (Fig. 5).
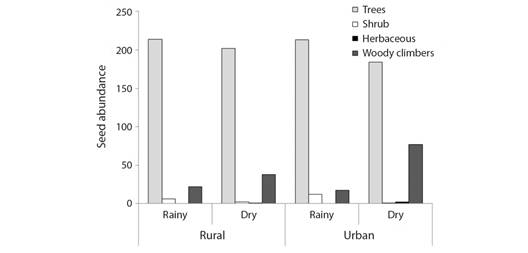
Fig. 5 Differences in seed abundance within the habit category (tree, shrub, herbaceous, and woody climbers) between Atlantic Forest fragments (rural and urban), and seasons (rain and dry).
There were two first-order interactions, between the fragments and dispersal syndromes (c2 = 26.11, df = 2; P < 0.0001) and between seasons and dispersal syndromes (c2 = 28.02, df = 2; P < 0.0001). It was also observed a second-order interaction between fragments, years, and seasons (c2 = 4.14, df = 1; P = 0.0418). These results also can be evaluated together. Although the total density of zoochoric seeds was higher than the seed density of the other syndromes, it was observed that the proportion of zoochoric, anemochoric, and autochoric seeds changes according to the fragment, season, and year. For example, the seed number of anemochoric species was higher in the dry season in the rural fragment in 2016; and in the dry season of the urban fragment in 2015 and 2016 (Fig. 6).
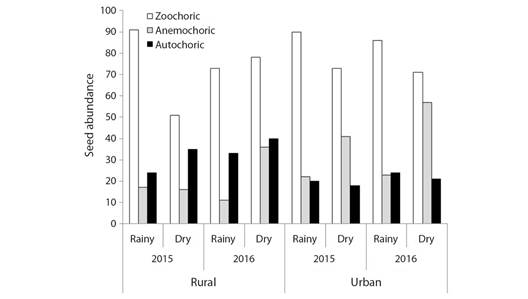
Fig. 6 Differences in seed abundance within the category of dispersal syndrome (zoochoric, anemochoric, and autochoric) between Atlantic Forest fragments (rural and urban), years (2015 and 2016), and seasons (rain and dry).
Finally, there was a first-order interaction between the year and successional category variables (c2 = 14.89, df = 2, P = 0.0006) and a second-order interaction between fragment, season, and successional category (c2 =11.45, df = 2, P = 0.0033). Additionally, evaluating all the variables together, it was observed that the proportion of early, secondary, and late secondary species changes according to the year, fragment, and season. For example, the seed number of secondary species was higher in 2015 than in 2016, however, the variation was higher in the rural fragment in 2016 (1.52 times more seeds in 2016) than in the urban one (1.06 times more seeds in 2016) (Fig. 7).
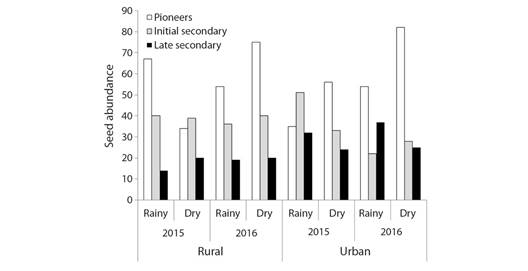
Fig. 7 Differences in seed abundance within the successional category (pioneer, early secondary, and late secondary - Gandolfi et al., 1995) between Atlantic Forest fragments (rural and urban), years (2015 and 2016), and seasons (rain and drought).
Discussion
Species richness, seed density and species composition: Our findings show that the urban fragment species richness was higher during the two years and this superiority is maintained due to the high number of species present during the rainy season. During the dry season, the urban fragment species richness reduces and equals the rural fragment species richness. Thus, the hypothesis of greater species richness in the rural fragment was not accepted. This behavior is opposite to that registered in the literature, where is a trend of greater species richness in rural fragments over time (Campos et al., 2009; Cutway & Ehrenfeld, 2010; Freitas et al., 2013; Knorr & Gottsberg, 2012; Overdyck & Clarkson, 2012).
The greater species richness in urban fragment can be explained by the peaks in the phenological patterns of flowering occur concentrated both in the rainy season (Morellato et al., 2013; Talora & Morellato, 2000), as in the dry-rainy transient period (Medeiros et al., 2007; Morellato et al., 2013); and fructification peaks, although not seasonally patterned, tend to occur more commonly in the rainy season (Medeiros et al., 2007; Morellato et al., 2013; Talora & Morellato, 2000). New studies are needed to test this hypothesis.
Our hypothesis of greater seed density in rural fragment was accepted. However, we verified that this superiority in not maintained over time. The high seed density in the rural fragment was a consequence of the high number of dispersed diaspores during the rainy season (Fig. 3). Therefore, the dry season contributed little to the differences in seed density between the fragments studied. The high seed density in the rural fragment can be a reflect of the increase in the number of vertebrates acting as dispersing agents due to the greater food availability, and the species phenology that makes up this area (Medeiros et al., 2007; Morellato et al., 2013; Oliveira et al., 2011). According to DeWalt et al. (2003), the abundance of plants with fleshy fruits acts as a quality indicator of habitat.
The compilation of the studies carried out in tropical rainforests, that were developed only in rural fragments or only in urban ones, showed a great variation in the seed density, so that in rural fragment (Cole et al., 2010; Freitas et al., 2013; Knorr & Gottsberg, 2012; Overdyck & Clarkson, 2012) the seed density was higher than recorded for urban forests (Campos et al., 2009; Cutway & Ehrenfeld, 2010; Overdyck & Clarkson, 2012; Pivello et al., 2006). When the seed density of rural and urban fragments inserted in the same landscape is compared, as in the case of this study, it is verified that the seed number remains superior in the rural fragment. Thus, we infer that the dynamics of soil use in the rural fragment studied, such as the use of fire, fertilizers, and pesticides, that occur in some months of the dry season, probably did not interfere in the action of pollinators and dispersants in the rainy season, which may have contributed to an increase in seed density of this fragment during the rainy season.
The floristic composition of the seed rain differed between the rural and urban fragments for two years, confirming the hypothesis of this study. In fact, the literature shows that surrounding matrix of rural and urban fragments may limit the dispersion of seeds and consequently, reduce the entry of allochthonous seeds. Over time, there is a growing trend in the number of unique species in each fragment, significantly increasing the dissimilarity (Cutway & Ehrenfeld, 2010; Overdyck & Clarkson, 2012; Pivello et al., 2006). In this sense, our results indicate that the differences in floristic composition between the fragments may have been in response to the process of urbanization and rise of agricultural frontiers. These processes promote a significant reduction in the ecosystem services of habitat and resource to wildlife, and microclimate for the establishment and growth of plants. Consequently, there may be a significant reduction in the pollination and dispersion of diasporas processes and, therefore, causing changes in floristic composition (Benítez-Malvido et al., 2022; Overdyck & Clarkson, 2012).
Functional Attributes: The variation in the functional attributes of habit, dispersal syndrome, and successional category were explained by the variable surrounding matrix of rural and urban fragments, confirming our hypothesis. In general, the rural and urban fragments presented a high density of tree seeds, zoochoric, and pioneer species when compared to the other habits, dispersal syndrome, and successional category. However, the proportion of these attributes of seed rain changed according to the studied fragment, with the rural one presenting a greater number of tree seeds, zoochoric, and pioneers than the urban.
It is probable that greatest quantity of seeds with these functional attributes in the rural fragment results from the phenology of the species that make up this area, and the great availability of fleshy fruits that are attractive to the dispersing animals. For the pioneer species, the high quantity of seeds may indicate that the forest has not reached maturity, or that maturity has been reached, but the presence of some natural or anthropic disturbance, such as the opening of clearings, has led to the appearance of a greater number of these species. The high representativeness of trees, zoochoric, and pioneer species corroborates the assumptions reported by several authors that in tropical rainforests (rural or urban) and specifically in the Atlantic Forest, there is a domain of tree habit, zoochoric dispersion, and early succession species (Cole et al., 2010; Marangon et al., 2010; Oliveira et al., 2011).
In addition, the opening of clearing can be contributed to an increase in the wind incidence and greater light input, easing the dispersion of anemochoric seeds and the emergence of pioneer species that produce small and light seeds (César et al., 2017; Plohák et al., 2021). Therefore, we infer that the canopy discontinuity due to the presence of clearings, as well as the increase in the velocity of the winds in the dry season of the studied urban fragment, may have favored the displacement of woody climbing plant seeds because they are small and because they are light.
Finally, our findings show that the species richness, seed density, and floristic composition were influenced by the surrounding matrix of rural and urban fragments over time. There was greater species richness in the urban fragment and higher seed density in the rural fragment. Seed input is more intense during the rainy season in the rural fragment and similar between seasons in the urban fragment. The species recorded in the seed rain of the rural fragment are not the same as those recorded in the urban fragment. During the two years of study, the rural and urban fragments presented a high seeds density of tree, zoochoric dispersal syndrome, and pioneer species.
Our results suggest that landscape disturbance measures can be effectively used to assess the impact of land use in seed rain studies, especially in urban and rural fragments. However, it is necessary to identify the cause-effect relationship of the impact from different uses of the surrounding soil, and the characteristics of the plants in larger time series. This investigation will be useful in the current and future management of remnants of rural and urban fragments. Future studies could sample more forest fragment in rural and urban conditions to identify process governing the seed rain.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgments section. A signed document has been filed in the journal archives.












 uBio
uBio 


