Introducción
The Caatinga is a tropical dry forest exclusively found in the Northeast of Brazil that exhibits high biodiversity, including endemic species. This biome covers about 845 000 km2 and is characterized by semi-arid climate, stony and shallow soils, and deciduous vegetation (Pinheiro & Nair, 2018). The Caatinga population includes approximately 28 million inhabitants and more than 40 % are small farmers who depend on natural resources for their livelihoods. Unfortunately, anthropic activities have severely disturbed the Caatinga vegetation, altering its structure, composition, and diversity (Specht et al., 2019). This scenario requires efforts focused on the restoration of degraded areas.
One of the main limitations for the restoration of tropical dry forests is the lack of knowledge on seed germination and seedling establishment of strategic species (Soriano et al., 2013). Seed germination consists of the resumption of embryo metabolism and growth, while seedling establishment involves the transition from heterotrophic metabolism to photosynthetic autotrophy (Bewley et al., 2013). These developmental transitions rely on the reserves deposited in the embryo axis and the storage tissues as sources of metabolic energy and building blocks (Gommers & Monte, 2018). Therefore, reserve mobilization plays a central role in seedling survival and fitness under natural conditions (Soriano et al., 2013).
Starch, oils, and storage proteins are reserves commonly accumulated in the storage tissues during seed development (Bewley et al., 2013). In desiccated seeds, these reserves are maintained in the form of insoluble aggregates within different cell compartments. When metabolism is reactivated by rehydration, reserves are degraded to generate soluble metabolites, which are transported to the embryo axis to support growth (Gommers & Monte, 2018). Despite the importance of this process for forest dynamics (Soriano et al., 2013), reserve mobilization is still poorly known in woody species, especially those from tropical dry forests.
The mobilization process is characterized only in a few woody species native to the Caatinga, like Caesalpinia pyramidalis (Dantas et al., 2008a), Schinopsis brasiliensis (Dantas et al., 2008b) and Anacardium occidentale (Voigt et al., 2009). Additionally, several studies focusing on reserve mobilization in species from other forest biomes do not clarify the involvement of hydrolytic enzymes in the heterotrophy-autotrophy transition (Corte et al., 2006; Paula et al., 2016; Veronesi et al., 2014; Weidlich et al., 2010). Considering these knowledge limitations, this work is an integrative approach on reserve mobilization, metabolite accumulation and hydrolase activity in the cotyledons of Erythrina velutina Wild. during seed germination and seedling establishment.
E. velutina is used as a model species for several reasons. It is a pioneer tree legume that naturally inhabits the Caatinga and is recommended to the restoration of degraded areas, since it exhibits fast growth, deep roots, drought tolerance, and the ability to establish symbiosis with nitrogen-fixing bacteria (Pereira et al., 2014; Ribeiro et al., 2018; Rodrigues et al., 2018). E. velutina also provides wood, is an ornamental plant, and presents medicinal properties due to the secondary metabolites it accumulates (Rambo et al., 2019; Ribeiro et al., 2018). Secondary metabolites include terpenes, phenolics, and alkaloids, which are non-essential for plant growth and reproduction, but play a part in signaling, defense, and response to environmental stresses (Pang et al., 2021). Although many studies have characterized the pharmacological properties of secondary metabolites from E. velutina, their role in seed germination and seedling establishment are still elusive. Considering that these compounds may contribute to seedling survival and fitness in natural environments (Chacón et al., 2013), a profile of these compounds is also assessed in parallel with reserve mobilization.
Materials and methods
Plant material: E. velutina seeds were harvested from five mother trees located at Acari, RN, Brazil (6°27'45'' S & 36°38'31'' W), packaged in paper bags and kept under refrigeration. Seeds were mechanically scarified in the region opposite to the hilum, immersed in commercial detergent at 1:500 (v/v) dilution for 30 s and rinsed under running tap water. Next, seeds were surface-sterilised with 70 % (v/v) ethanol for 30 s followed by 0.25 % (v/v) NaClO for 3 min, washed three times and imbibed in sterile distilled water for 2 h. Seeds were sown between towel paper sheets moistened with 2.5 mL of distilled water per g of dry paper. Paper sheets were rolled up and covered with transparent plastic bags. Paper rolls were maintained upright under controlled conditions (photosynthetic photon flux density of 80 μmol m-2 s-2, 12 h light/12 h dark photoperiod and 27 ± 2 °C) for 9 days (International Seed Testing Association, 2006).
Seven-day-old seedlings were transferred to plastic pots containing distilled water and were cultivated in a greenhouse for 8 days. In an attempt to assess how the heterotrophy-autotrophy transition takes place when the seedlings rely only on their reserves, nutrient solution was not used in the hydroponic system. Seedlings exhibiting apparent nutrient deficiencies or morphological abnormalities were eliminated. The harvests were performed at seven different morphological stages (Fig. 1): imbibed seed (stage I), radicle protrusion (stage II), hypocotyl emergence (stage III), apical hook formation (stage IV), and expansion of cordiform leaves (stage V), first trifoliate leaf (stage VI) and second trifoliate leaf (stage VII). At every harvest, the cotyledons were weighed and frozen at -20 °C until biochemical quantifications.

Fig. 1 Morphological stages of Erythrina velutina during seed germination and seedling establishment: A. Seed imbibition. B. Radicle protrusion. C. Hypocotyl emergence. D. Apical hook formation. E. Expansion of cordiform leaves. F. Expansion of first trifoliate leaf. G. Expansion of second trifoliate leaf.
Biochemical quantifications: Neutral lipids were quantified by the gravimetric method (Soxhlet, 1879). Samples containing 300 mg of dry cotyledons were extracted with 8 mL of n-hexane at 60 °C for 5 h under intermittent stirring. The extracts were collected and transferred to tubes whose mass was previously weighed. After the evaporation of n-hexane at 80 ºC, the mass of neutral lipids was calculated as the difference between the initial and final mass of the tubes and expressed as mg cotyledon-1.
To obtain an extract enriched in storage proteins, samples of 300 mg of frozen cotyledons were extracted by maceration with 1.5 mL of 100 mM Tris-HCl buffer pH 7.0 supplemented with 500 mM NaCl and 2 mM 2-mercaptoethanol (Barros-Galvão et al., 2017). The homogenates were centrifuged at 10 000 xg for 10 min, the supernatants were collected, and pellets were re-extracted twice with 1 mL of the extraction buffer, yielding 3.5 mL of extract per sample. These procedures were performed at 4 °C. Soluble proteins were determined according to the Bradford (1976) method, using bovine serum albumin as standard. The content of soluble proteins was expressed as mg cotyledon-1.
To extract the soluble metabolites, samples containing 300 mg of frozen cotyledons were fragmented and extracted with 5 mL of 80 % (v/v) ethanol at 60 °C for 30 min. The supernatants were collected, and the residuals were re-extracted two times with 5 mL of 80 % (v/v) ethanol under the same conditions, yielding 15 mL of ethanolic extract per sample. Total soluble sugars (TSS) were measured with the anthrone reagent (Morris, 1948; Yemm & Willis, 1954), employing a D-glucose standard curve. Non-reducing sugars (NRS) were quantified by a modification of the anthrone method (Van Handel, 1968), using sucrose as standard. Total free amino acids (TFAA) were determined by the ninhydrin reagent (Yemm & Cocking, 1955), utilizing a L-glutamine standard curve. The contents of all these metabolites were expressed as μmol g-1 dry weight (DW).
Starch was extracted from the residuals obtained after the removal of ethanol-soluble metabolites. The residuals were macerated with 1.5 mL of 30 % (v/v) HClO4 and the homogenates were centrifuged at 10 000 xg for 10 min. The supernatants were collected, whereas the pellets were re-extracted twice with 1 mL of 30 % (v/v) HClO4, yielding 3.5 mL of extract per sample. These steps were carried out at 4 °C. Starch was measured with the anthrone reagent (Morris, 1948; Yemm & Willis, 1954), using D-glucose as a standard. The values were multiplied by 0.9 (McCready et al., 1950) and the content of starch was expressed as mg cotyledon-1.
Enzymatic activities: The extraction of amylases was carried out by maceration of 300 mg of frozen cotyledons with 1.5 mL of 100 mM potassium acetate buffer pH 6.0 containing 5 mM CaCl2. The samples were centrifuged at 10 000 xg for 20 min and the supernatants were employed as sources of enzymes. These steps were carried out at 4 °C. Amylase activity was assayed by the release of reducing sugars from soluble starch (Elarbi et al., 2009). The concentration of reducing sugars in the reaction medium was quantified by the 3,5-dinitrosalicylic acid method (Miller, 1959), using a D-glucose standard curve. The activity was expressed as μmol reducing sugars g-1 DW min-1.
Lipases were extracted from samples of 300 mg of frozen cotyledons, which were macerated with 1.5 mL of 50 mM potassium phosphate buffer pH 7.5 supplemented with 0.01 % (v/v) 2-mercaptoethanol. After centrifugation at 10 000 xg for 20 min, the supernatants were collected and used as enzymatic extracts. All procedures were performed at 4 °C. Lipase activity was estimated by the hydrolysis of 4-nitrophenyl-palmitate, generating 4-nitrophenol in the reaction medium (Marriot & Northcote, 1975). The activity was expressed as μmol 4-nitrophenol g-1 DW min-1.
For the extraction of acid proteases, 300 mg of frozen cotyledons were macerated with 1.5 mL of 50 mM Tris-HCl buffer pH 7.2 containing 2 mM 2-mercaptoethanol. The homogenates were centrifuged at 10 000 xg for 20 min and the supernatants were utilized as a source of enzymes. These procedures were carried out at 4 ° C. Acid protease activity was estimated by the liberation of amino acids from casein (Beevers, 1968). The concentration of free amino acids in the reaction medium was measured with the ninhydrin reagent (Yemm & Cocking, 1955), utilizing L-glutamine as a standard. The activity was expressed as μmol free amino acids g-1 DW min-1.
Thin-layer chromatography (TLC): Samples of 180 mg of frozen cotyledons were solubilized in 1.8 mL of 80 % (v/v) ethanol and maintained in an ultrasound bath at 45 °C for 30 min. The samples were centrifuged at 16 000 xg for 10 min at 25 °C, the supernatant was transferred to another tube and the residual was dried by SpeedVac for 5:30 h. The final mass of the sample was assessed by gravimetry and solubilized in 300 μl of 70 % (v/v) ethanol. Samples were submitted to TLC by application in silica gel 60GF254 normal phase chromatoplates. The mobile phase was a mixture of ethyl acetate, formic acid, acetic acid, and water in the proportions of 10:1.1:1.1:2.6 (v/v/v/v). After elution and observation under UV light at 254 nm, the staining was performed with vanillin/sulfuric acid, Natural A and the Dragendorff reagent (Izmailov & Schraiber, 1938).
Experimental design and statistical analysis: During the experiment, samples of cotyledons were randomly harvested at each physiological stage. The experimental unit corresponded to one seedling. The biochemical quantifications were performed using four replicates, whereas the qualitative analysis of secondary metabolites was carried out using three replicates. As the seedlings did not exhibit synchronized growth, the harvests were not performed at regular time intervals. Considering the physiological stages as categories, the data were submitted to variance analysis and the means were compared by the Tukey test at 5 % of confidence.
Results
Significant contents of starch (Fig. 2A), neutral lipids (Fig. 2B) and soluble proteins (Fig. 2C) were detected in the seeds of E. velutina. On a DW basis, the seeds contained approximately 20 % starch, 11.6 % neutral lipids and 14.5 % soluble proteins at the beginning of the experiment. Besides these major reserves, the seeds also presented a minor content of NRS (Fig. 3B), equivalent to 5.7 % of the DW.
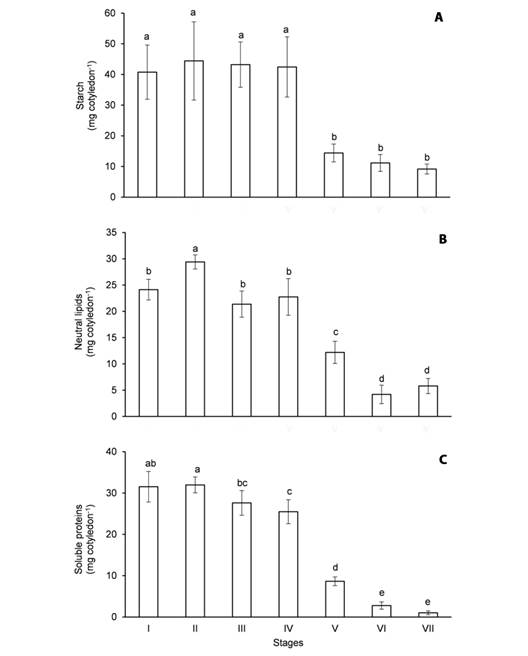
Fig. 2 Content of major reserves in the cotyledons of Erythrina velutina during seed germination and seedling establishment. A. Starch. B. Neutral lipids. C. Soluble proteins. Columns represent means and error bars correspond to standard deviation of four repetitions. Values marked with the same letter does not significantly differ according to the Tukey test at 5 % of confidence.
The content of starch (Fig. 2A) in the imbibed seeds (stage I) was 40.7 mg cotyledon-1 and it was not significantly changed until apical hook formation (stage IV). Starch was intensely mobilized in the cotyledons from stage IV to the expansion of the cordiform leaves (stage V) since its content decreased 64 % during this period. From then on, starch was gradually mobilized, as its content was 14.4 mg cotyledon-1 at stage V and it dropped to 9.1 mg cotyledon-1 at the expansion of the second trifoliate leaf (stage VII).
The content of neutral lipids (Fig. 2B) and soluble proteins (Fig. 2C) in the imbibed seeds was 24.1 and 31.5 mg cotyledon-1, respectively, and it remained almost unaffected until apical hook formation. The mobilization of oils in the cotyledons was clearly verified from stage IV to the expansion of the first trifoliate leaf (stage VI), as the content of neutral lipids decreased 82.4 % during this period (Fig. 2B). Like the mobilization of starch, the hydrolysis of storage proteins in the cotyledons was intensified from stage IV to V and then occurred gradually until stage VII. Indeed, the content of soluble proteins decreased 73 % from the first harvest to the expansion of the cordiform leaves and only 3.2 % of the initial content remained at the last harvest (Fig. 2C).
Although the mobilization of the major carbon reserves (starch and oils) was evidenced at stage V (Fig. 2A, Fig. 2B), the utilization of soluble sugars in the cotyledons took place earlier, at stage IV. In the imbibed seeds, the content of TSS (Fig. 3A) and NRS (Fig. 3B) was 558.5 and 172.3 μmol g-1 DW, in that order, and it was not significantly altered until stage III. However, the content of TSS and NRS in the cotyledons decreased 50 and 69.5 %, respectively, from hypocotyl emergence to apical hook formation. It is noteworthy that the content of these metabolites remained unchanged in the cotyledons during the mobilization of starch (Fig. 2A) and oils (Fig. 2B).
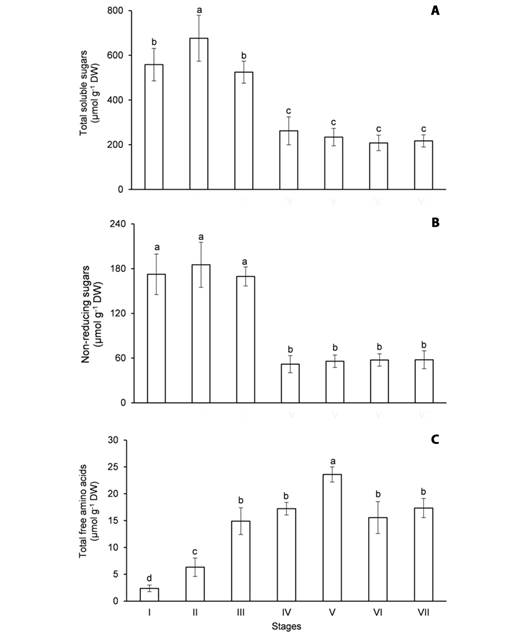
Fig. 3 Content of soluble metabolites in the cotyledons of Erythrina velutina during seed germination and seedling establishment. A. Total soluble sugars. B. Non-reducing sugars. C. Total free amino acids. Columns represent means and error bars correspond to standard deviation of four repetitions. Values marked with the same letter does not significantly differ according to the Tukey test at 5 % of confidence.
Different from soluble sugars, the content of TFAA increased in the cotyledons during seed germination and seedling establishment (Fig. 3C). The content of TFAA was 2.37 μmol g-1 DW in the imbibed seeds and it increased 2.6 times at radicle protrusion (stage II). Coincidently, TFAA were accumulated when the hydrolysis of storage proteins was intensified (Fig. 2C). In fact, the content of these metabolites increased 3.7 times from stage II to V and decreased 27 % from stage V to VII (Fig. 3C).
The activity of amylases (Fig. 4A), lipases (Fig. 4B) and acid proteases (Fig. 4C) in the cotyledons increased during the experiment, especially when starch (Fig. 2A), oils (Fig. 2B) and storage proteins (Fig. 2C) were intensely mobilized. In the first harvest, the amylase activity was 11.5 μmol g-1 DW min-1, it doubled from stage I to V and a 2.3-fold increase was noticed from stage V to VII (Fig. 4A). The lipase activity was 22.4 μmol g-1 DW min-1 in the imbibed seeds, it oscillated from stage I to IV and it increased 4.5 times from stage IV to VII (Fig. 4B). By contrast, the acid protease activity was only 0.068 μmol g-1 DW min-1 at the beginning of the experiment, a 14-fold increase was verified from stage I to VI and it decreased 48 % from stage VI to VII (Fig. 4C).
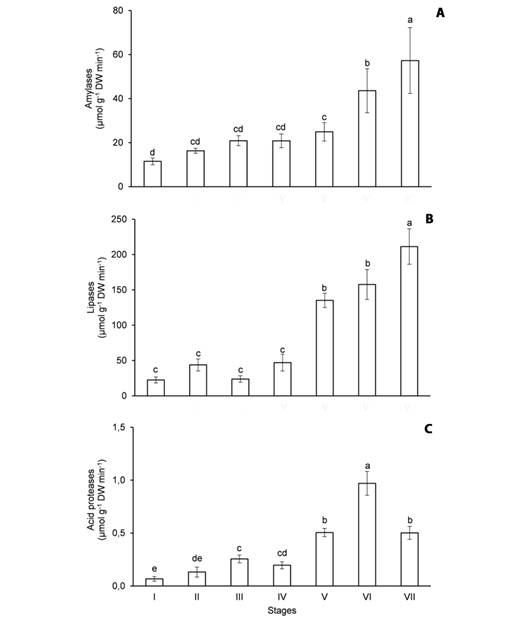
Fig. 4 Activity of hydrolytic enzymes in the cotyledons of Erythrina velutina during seed germination and seedling establishment. A. Amylases. B. Lipases. C. Acid proteases. Columns represent means and error bars correspond to standard deviation of four repetitions. Values marked with the same letter does not significantly differ according to the Tukey test at 5 % of confidence.
When the ethanolic extracts obtained from cotyledons were submitted to TLC, it was possible to verify that the profile of secondary metabolites changed during seed germination and seedling establishment (Fig. 5). Vanillin/sulfuric acid staining suggested the occurrence of terpenoid-derivative metabolites (brown dots) in the seeds at Rf 0.21-0.27 and these metabolites were detected mainly until apical hook formation (Fig. 5A). According to Natural A staining, flavonoids (yellow dots) and phenolic acids (blue dots) were present in almost all stages, but chlorophylls (red dots) were detected only from stage V to VII. (Fig. 5B). The Dragendorff reagent indicated that the seeds also contained alkaloids (orange dots) (Fig. 5C). Curiously, an alkaloid (RF: 0.4 (I)) disappeared at radicle protrusion and was slightly detected at hypocotyl emergence and the expansion of the cordiform leaves. Otherwise, an alkaloid spot was detected in all samples near to application point (Rf < 0.1) and it remained throughout seed germination and seedling establishment. The exact number of alkaloids in this spot and their chemical identities remain to be investigated.
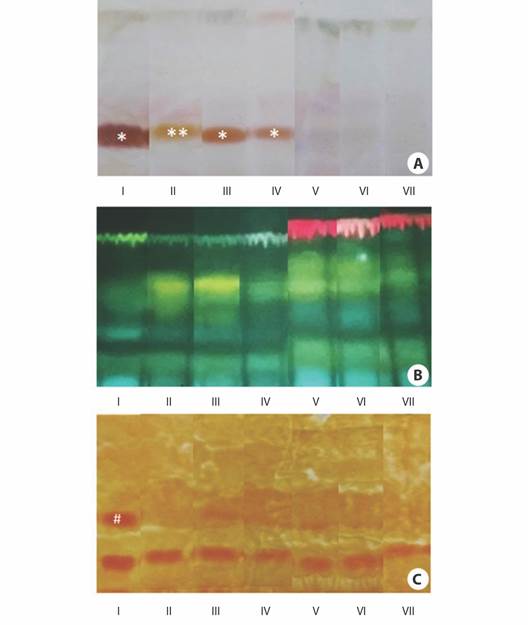
Fig. 5 Thin-layer chromatograms of ethanolic extracts obtained from the cotyledons of Erythrina velutina during seed germination and seedling establishment. A. Staining with vanillin/sulfuric acid for terpenoid-derivatives (brown dots-*Rf: 0.21 (I, III, IV), **Rf: 0.27 (II)) and other steroids (violet dots). B. Staining with Natural A for flavonoids (yellow dots), phenolic acids (blue dots), and chlorophylls (red dots), and C. Staining with the Dragendorff reagent for alkaloids (orange dots-#Rf: 0.40 (I)).
Discussion
The seeds of tree legumes native to the Caatinga are still poorly characterized in terms of nutritional reserves and only a few efforts have been made to evaluate the potential use of these seeds as food, feed, and industrial raw material (Carvalho et al., 2011; Mayworm et al., 1998). Edible legume seeds are commonly classified into pulses, which are almost devoid of lipids, and oilseeds, which are poor in starch (Vijayakumar & Haridas, 2021). As E. velutina seeds contain approximately 20 % starch, 11.6 % oils and 14.5 % storage proteins in a DW basis, they cannot be classified according to these categories. The seeds of other tree legumes also demonstrate balanced contents of starch and oils, like Caesalpinia bracteosa and Pterogyne nitens (Carvalho et al., 2011). Although the content of storage proteins in E. velutina seeds is similar to that found in Poecilanthe ulei (Mayworm et al., 1998) and C. bracteosa (Carvalho et al., 2011), the seeds of several tree legumes native to the Caatinga contain more than 30 % storage proteins (Carvalho et al., 2011; Mayworm et al., 1998). The diversity and proportion of major reserves stored by E. velutina seeds may be related to metabolic flexibility during the heterotrophy-autotrophy transition.
In addition to the major reserves, E. velutina seeds also contain nearly 5.7 % NRS on a DW basis. These compounds generally include sucrose and raffinose family oligosaccharides, which are considered as minor reserves. NRS may contribute to the longevity of orthodox seeds by stabilizing the glassy state and the membrane structure under desiccation (Bewley et al., 2013) and they probably play a role as immediate sources of respiratory substrates during seed germination (Rosental et al., 2014). The content of NRS in E. velutina seeds is comparable to that verified for other species, which varies from 2 to 6 % of the DW (Bewley et al., 2013).
As a pioneer species, E. velutina produces high quantity of large and viable seeds every year (Ribeiro & Dantas, 2018). In this work, it is verified that approximately 52 % of the seed DW correspond to nutritional reserves. Therefore, the size and reserve content of E. velutina seeds may be functional traits, since it is expected that large seeds containing abundant reserves are able to produce vigorous seedlings, which are likely to growth and survive under adverse conditions (El-Keblawy et al, 2018). Considering that environmental stresses such as water deficit and high temperature are commonly found in the Caatinga (Pinheiro & Nair, 2018), E. velutina may take advantage of these seed functional traits to enable successful seedling establishment.
As the content of starch (Fig. 2A), neutral lipids (Fig. 2B), and soluble proteins (Fig. 2C) in the cotyledons remain almost unaffected from seed imbibition (stage I) to radicle protrusion (stage II), there is no clear evidence that the mobilization of major reserves starts during the germination of E. velutina seeds. These results corroborate the notion that the mobilization of major reserves is a post-germinative event (Bewley et al., 2013). Like E. velutina, the mobilization of oils and storage proteins starts after seed germination in Caesalpinia peltophoroides (Corte et al., 2006), the content of soluble proteins remains unchanged in germinating seeds of Aniba rosaeodora (Lima et al., 2008) and starch mobilization is not detected during seed germination in Enterolobium contortisiliquum (Veronesi et al., 2014). Although the reserves stored in the embryo axis are not measured in this study, it is likely that they have sustained the germination of E. velutina seeds. In some species, the embryo axis stores low contents of sucrose, raffinose family oligosaccharides, oils, and storage proteins. It is accepted that the hydrolysis of these reserves during metabolism reactivation provides substrates for respiration and amino acids for protein synthesis (Bewley et al., 2013; Rosental et al., 2014).
In the cotyledons of E. velutina seedlings, the carbon and nitrogen reserves are simultaneously mobilized from mid to late establishment (Fig. 2), since the degradation of starch (Fig. 2A), oils (Fig. 2B), and storage proteins (Fig. 2C) is intensified from apical hook formation to the expansion of the first trifoliate leaf. It is well established that the hexoses released by starch degradation (Dong & Beckles, 2019) and the fatty acids resulting from oil hydrolysis (Theodoulou & Eastmond, 2012) are converted to sucrose for export to the seedling axis. It is also clear that the amino acids liberated by storage protein hydrolysis support protein synthesis in the storage tissues and are converted to amides for export to the growing tissues (Bewley et al., 2013). However, the metabolic processes responsible for the mobilization of different reserves in the cotyledons of E. velutina seedlings may be interdependent, considering that they occur simultaneously. Previous reports demonstrate that fatty acids and amino acids are alternative respiratory substrates and carbon skeletons derived from fatty acid degradation may be used for amino acid synthesis during the heterotrophy-autotrophy transition (Borek et al., 2015; Hildebrandt et al., 2015). If this metabolic flexibility occurs in E. velutina, it possibly contributes to seedling fitness under stresses.
It is noteworthy that the utilization of soluble sugars precedes the mobilization of major reserves in the cotyledons of E. velutina seedlings. As the content of TSS (Fig. 3A) and NRS (Fig. 3B) decreases from stage III to IV, soluble sugars may have supported early seedling establishment. Accordingly, NRS like sucrose and raffinose family oligosaccharides are more accessible reserves than starch and oils (Rosental et al., 2014). In the woody species Peltophoprum dubium (Veronesi et al., 2014) and Morinda citrifolia (Paula et al., 2016), seedling growth also initiates at the expense of soluble sugars provided by the storage tissues.
The mobilization of starch (Fig. 2A) and oils (Fig. 2B) does not result in accumulation of soluble sugars (Fig. 3A and B) in the cotyledons of E. velutina seedlings from mid to late establishment. Similarly, the mobilization of carbon reserves does not increase the content of soluble sugars in the cotyledons during seedling establishment in the tree legumes C. peltophoroides (Corte et al., 2006) and Schizolobium parahyba (Weidlich et al., 2010). As a developmental transition, seedling establishment involves alterations in the source-sink relationship, since the growth of new organs generates additional carbon sinks (Wingler, 2018). In E. velutina seedlings, the axis sink strength may have predominantly increased at stage V, when the expansion of the cordiform leaves coincides with the growth of secondary roots. Given that soluble sugars are the major products of starch and oil degradation (Dong & Beckles, 2019; Theodoulou & Eastmond, 2012), these metabolites may have been translocated to the seedling axis to support its growth.
The mobilization process involves the coordinated action of several enzymes inside different cell compartments in the storage tissues. Amylases are essential for the dismantling of starch granules in amyloplasts (Bewley et al., 2013), lipases are responsible for the hydrolysis of triacylglycerols in oleosomes (Borek et al., 2015) and acid proteases catalyse the first cleavages of protein aggregates in storage vacuoles (Tan-Wilson & Wilson, 2012). In the cotyledons of E. velutina seedlings, the activity of amylases (Fig. 4A), lipases (Fig. 4B) and acid proteases (Fig. 4C) simultaneously increases from mid to late establishment, enabling the mobilization of starch (Fig. 2A), oils (Fig. 2B), and storage proteins (Fig. 2C). Although only a few works have evaluated the activity of hydrolases during reserve mobilization in other woody species, the activity of amylases and acid proteases also increases after seed germination in A. rosaeodora (Lima et al., 2008) and Pistacia vera (Einali & Valizadeh, 2017), respectively. Curiously, an increase in the activity of reserve-degrading enzymes (Fig. 4) coincides with a decrease in the content of soluble sugars (Fig. 3A, Fig. 3B) and an accumulation of TFAA (Fig. 3C) in the cotyledons of E. velutina seedlings. Considering that carbon availability is involved in the regulation of seedling metabolism (Wingler, 2018), it is possible that decreased sugar supply plays a part in inducing the activity of hydrolases. Additionally, amino acid accumulation could be related to enhanced protein turnover in the cotyledons (Tan-Wilson & Wilson, 2012).
Interestingly, the profile of secondary metabolites in the cotyledons of E. velutina changes during seed germination and seedling establishment (Fig. 5). As previously reported for the genus Erythrina (Rambo et al., 2019), terpenoid-derivatives (Fig. 5A), flavonoids, phenolic acids (Fig. 5B), and alkaloids (Fig. 5C) are detected by TLC. Even though the roles of secondary metabolites in early development are still poorly investigated, it is proposed that they are involved in seedling defence (Pang et al., 2021). Since flavonoids and phenolic acids are found at almost all stages (Fig. 5B), they might play a part as a constitutive defence against pathogens (Chacón et al., 2013). By contrast, terpenoid-derivatives are detected only during seed germination and early seedling establishment (Fig. 5A). The universal stain vanillin/sulfuric acid used in TLC does not reveal the exact class of terpenoid-derivatives present in the samples. Among these compounds, the occurrence of saponins and steroids may be the most plausible. Like flavonoids, terpenoid-derivatives could also be involved in seedling defence as they demonstrate antifungal activity (Naboulsi et al., 2018).
In addition to the secondary metabolites mentioned above, an alkaloid dot is found throughout the experiment, while another disappears at radicle protrusion (Fig. 5C). It has recently been demonstrated the occurrence of erythraline and erysodine in the seeds used herein, which are alkaloids found in the embryo, testa and mainly in the cotyledons (unpublished data). Considering that major reserves (Fig. 2) and soluble sugars (Fig. 3A, Fig. 3B) are not degraded, TFAA are accumulated (Fig. 3C), and some alkaloids disappear (Fig. 5C) in the cotyledons of E. velutina during seed germination, these alkaloids might play a role as nitrogen reserves during seed germination (Chacón et al., 2013). Alternatively, they could also exhibit allelopathic activity (Oliveira et al., 2012), contributing to seedling fitness during early establishment.
According to the results, it is possible to conclude that carbon and nitrogen reserves stored in the cotyledons of E. velutina are mobilized after seed germination. Soluble sugars support early seedling growth, while starch, oils, and storage proteins are utilized from mid to late establishment. Major reserves are simultaneously mobilized by the coordinated action of amylases, lipases, and acid proteases, especially during the expansion of the first leaves. E. velutina seeds contain terpenoid-derivatives, flavonoids, phenolic acids, and alkaloids, which may play a part in seedling defense during germination and establishment. The high content of reserves and the presence of secondary metabolites in the cotyledons could provide E. velutina seedlings with the ability to survive stresses, supporting their use in the restoration of degraded areas.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgments section. A signed document has been filed in the journal archives.












 uBio
uBio 


