Introduction
Amazona aestiva (Linneaus, 1758), known as the turquoise-fronted parrot, is a species of Neotropical parrot widely distributed in ecosystems in Brazil such as humid forests, caatinga, and cerrado (Sick, 2001). Although the species is considered of ''Near Threatened '' for the risk of extinction (IUCN, 2018), the population has been gradually decreasing due to habitat loss and, mainly, by illegal trade (Schunck et al., 2011). In Brazil, Amazon aestiva is one of the psittacids that most often enter wildlife screening centers (Destro et al., 2012; Schunck et al., 2011). Therefore, decisions on how these rescued animals are handled must be judicious, while also ensuring their safe return to nature (White Jr. et al., 2012).
Wildlife reintroductions are common conservation tools aimed at reestablishing populations of species within their historic geographic range (Armstrong & Seddon, 2008; Fischer & Lindenmeyer, 2000; Griffith et al., 1989; White Jr. et al., 2012). Reintroduction projects of Amazona aestiva help determine the destination of animals rescued from trafficking and provide more accurate information on procedures on this methodology, which can be applied to the conservation of other threatened species (de Azevedo et al., 2017; Silva et al., 2021; White Jr. et al., 2012).
According to a systematic review that evaluated 75 scientific studies on bird reintroductions and the threats to restoring these populations, 13 factors had a negative impact on these activities. The main factors were predation and unexpected dispersion of animals (Destro et al., 2018).
A strategy used to ensure the fidelity of reintroduced animals is supplementary feeding with feeders and artificial nests placed in the release areas (Tollington et al., 2013; White Jr. et al., 2012). Long-term food supplementation can help increase social interactions and improve the integration of new birds reintroduced into previously established flocks (Brightsmith et al., 2005; Elliott, 2006; Lima et al., 2014; Paranhos et al., 2007; Plair et al., 2008).
The characteristics and state of conservation of the environment, as well as climatic conditions, can also influence the fidelity of reintroduced animals. Physical environmental factors define microclimates occupied by birds and these microclimates affect energy balance and water consumption and influence behaviors (Bakken et al., 1991; Petit et al., 1985; Ryder, 1977; Wolf & Walsberg, 1996). Moreover, the combination of these factors may affect how reintroduced birds search for supplementary feeding and may influence the fidelity of animals to the area. Therefore, it is critical to understand how environmental factors can interfere with the search for supplementary feeding in feeders when managing areas destined for reintroduction. This information can help managers decide the best way to implement, maintain, and monitor feeders (Ewen et al., 2015).
Supplementary feeding is associated with many positive effects in various bird reintroduction projects (Brightsmith et al., 2005; Vilarta et al., 2021). However, further research is needed on the broader impacts of food supply and factors such as climate, pathogen dispersal, and predation (Robb et al., 2008). Thus, this study aimed to evaluate the reintroduction success of a group of Amazona aestiva and whether abiotic factors (temperature, humidity, and luminosity) interfere in the daily search dynamics for food supplementation in feeders. The hypothesis is that animals may seek more food supplementation in more unfavorable abiotic conditions for more distant foraging, such as low light, temperature, and high humidity.
Materials and methods
Animal: At the beginning of this study (January 2015), the Wildlife Screening Center (Centro de Triagem de Animais Silvestres - CETAS, in Portuguese) in Vitória da Conquista, housed 59 Amazonas spp. These animals came mainly from illegal trade confiscations. This study was submitted to and approved by the Animal Ethics Committee of the Multidisciplinary Institute of Health of the Universidade Federal da Bahia, under number 034/2015.
All 59 animals were initially tested to detect gastrointestinal parasites. For this test, fecal samples were randomly collected from the floor of the enclosures according to the number of birds. Stool parasitological tests were performed using the direct method, the Willis method, and the spontaneous sedimentation method (Matos & Matos, 1988). Once the endoparasites were identified, all birds were subjected to therapeutic interventions with piperazine citrate tetrahydrate, diluted in water, for three consecutive days (Miranda et al., 2014). New fecal samples were collected to evaluate the effectiveness of treatment after 20 days.
Soon after completing the first dose of antiparasitic treatment, flight capacity was evaluated only for animals of the species Amazona aestiva, the focus of this study. This evaluation was carried out using the methodology proposed by Pedroso (2013), in which the flight capacity of each animal is related to a score ranging from 1-4, from animals that did not fly to those that flew with constant rhythm and height. Only animals with a score of three or four had the flight capacity required to continue in the reintroduction project.
The animals selected in the flight capacity test were transferred to a single enclosure 4.15 m in length, 3.70 m in width, and 4.00 m in height outside the CETAS building, near denser vegetation, used to prepare animals for reintroduction. After three days of acclimatization, the test of socialization/aversion to humans was performed. Animals that showed aversion to the approach of an unknown human offering food were subsequently selected. The animals that accepted the food were removed from the enclosure and returned to the initial enclosures (Pedroso, 2013; Ramos et al., 2021).
From the selected group, feces were collected again from the enclosure to evaluate the effectiveness of the treatment, as previously described. Blood samples were also collected to analyze hematological parameters (leukogram) and for molecular sexing. The blood was collected by cutting the claw to obtain a small volume of blood (around 10 µl), according to Echols (1999), Murray (1997), and Silva et al. (2021). Blood smears were immediately prepared by the sliding technique and Panoptic fast staining to assess the leukocyte profile of the animals (Guzman et al., 2008).
A drop of blood from each bird was transferred to a filter paper for molecular sexing using an optimized protocol described by Griffiths (1998) and published by Barros et al. (2017) to determine the ratio between released males and females and support the reproductive monitoring of the established pairs. At the time of blood collection, all animals were individually clinically evaluated by a veterinarian to detect clinical signs of any infectious disease that was suggestive of further research (Miranda et al., 2014).
Environmental physical and food enrichment was used to support the social interaction between individuals seeking group cohesion, flight training, stimulation for the formation of pairings, and to stimulate post-release foraging. The enrichment was carried out in the CETAS facilities for four months using tree branches, hanging toys made with colored wood and sisal rope, and whole fruits hanging or hidden in boxes. Enrichment elements were changed twice a week (Rupley & Simone-Freilicher, 2015; Simone-Freilicher & Rupley, 2015).
Each animal was identified with a numbered metal ring placed on the tarsus (Rings-CETAS/UFBA + numbering) and by microchip (electronic microchip, microchip-electronic tag in the 12 mm mark) with individual numbers inserted into the pectoral muscle of the animal without impairing its flight capacity. In addition to these markings, the ventral region of the animals was painted with non-toxic red paint for easier identification in the wild, after reintroduction (Raigoza-Figueras, 2014). To facilitate distance identification, photographs of the animals, specifically the phenotypic characteristics of the head region, were taken of each individual and included in a portfolio.
Study area: The area selected to receive the animals is in the rural area of the municipality of Condeuba, Bahia, Brazil, at an altitude of approximately 634 m (14°53'43'' S & 41°48'11'' W). Condeuba has approximately 17 000 inhabitants and an area of 1 285.9 km², with a population density of 13.1 inhabitants per km², according to the regional census (Cidade-Brasil, 2016).
The area is characterized by vegetation typically found in the transitional environment of two biomes, that is, medium and large trees such as Copaifera langsdorffii Desf. (Caesalpinioideae) in the Atlantic Forest and vegetation such as Anadenanthera macrocarpa in the Caatinga (Benth.) (IBGE, 2016). According to the European Centre for Medium-Range Weather Forecasts (ECMWF), the highest humidity (68.02 %) and rainfall (average of 132 mm) in the region is recorded in December. In contrast, the lowest humidity, with 54.30 %, is recorded in September. November has the highest number of rainy days (12.10 days), while August is the dried month, with 1.63 days and 6 mm. The average annual temperature in Condeuba is 22.9 °C. The warmest month of the year is February, with an average temperature of 24.5 °C. The lowest temperature of the year is in July, with an average temperature of 20.2 °C. January has the most daily hours of sunshine with an average of 8.17 hours. Average annual rainfall is 630 mm (climate-data.org, 2022).
The release site includes a permanent preservation area of native forest with three sources of a permanent river. One of the main criteria used for choosing this area was its location, as it is of difficult access and with few signs of human activity, composed predominantly of small farmers, and the state of forest preservation. Another important criterion was the reports of older residents on the occurrence of native populations of Amazona aestiva and the identification by the CETAS team of other psittacine species in the area, such as Eupsittula cactorum and Eupsittula aurea.
To ensure the reintroduced animals remain close to the chosen release area, they were kept in installed nurseries for 10 days (Mitchell et al., 2011). During this period, the parrots received fruits and seeds collected in the locality, as well as sunflower seeds that were routinely used for food during their captivity in CETAS. The parrots received prophylactic treatment to eliminate the risk of contamination by gastrointestinal parasites during their transport to the reintroduction site. For this treatment, mebendazole (Avitrin®, Coveli) was used, in the dilution of 2.4 ml (containing 0.12 g of the active ingredient) in 1 l of water. The dilution was left in the enclosure in a single collective drinker freely accessed by the animals for three consecutive days and changed daily. The dilution was administered according to the manufacturer's guidelines.
Reintroduction: The animals were reintroduced using the soft-release method (Macias et al., 2010). During release, the nurseries were opened in the morning and the spontaneous exit of the animals was monitored and recorded. At the end of the day, the nurseries were closed to prevent predators from entering at night. In the morning in the following days, the animals that had remained in the avian nursery were identified and the doors were opened again (de Oliveira et al., 2014; Silva et al., 2021). This procedure was repeated for one month (the period in which the enclosures remained in the area), and animals that did not leave during the period in which the doors remained open were always identified.
After the nurseries were opened, the supplementary diet of regional fruits and sporadic sunflower seeds (the same seeds offered during the acclimatization period) was provided twice a day inside the nurseries and in a feeder installed 5 m from the avian nursery. In the study area, 12 artificial nests were also installed to encourage reproduction, as performed by White Jr. et al. (2005).
Monitoring: Monitoring was divided into two stages, from May 2015 to May 2016. In the first stage, the animals were evaluated for 18 consecutive days immediately after release, while in the second stage, they were evaluated for three to seven days at intervals of two months, during a year, totaling six monitoring campaigns. Monitoring was carried out at dawn and in the late afternoon (for 2 hours per period) to identify the animals around the release site (in trees) and the animals feeding in the feeders. When an animal was seen feeding on native fruits scattered in the study area, the plant species were also identified (Silva et al., 2021).
The animals were identified through the microchip reader or by detecting the ring or phenotypic characteristics recorded in the portfolio (Silva et al., 2021). Binoculars and cameras were used to record the sightings (de Almeida & de Almeida, 1998). In addition to these monitoring stages, between the two daily observations, the neighbors of the release site were visited and questioned about the possible presence of the reintroduced birds on their properties.
To identify the paired animals, mutual care behaviors such as beak to beak, cleaning care, sleeping, and feeding close, and moving together in the departures and arrivals in the monitored area were observed (Trillmich, 1976a; Trillmich, 1976b). Pairing was considered when the animals formed a pair for at least two monitoring campaigns. Each installed artificial nest was inspected during the campaigns to identify possible occupations. Nests that showed signs of occupation were identified and photographed.
Abiotic factors were evaluated according to the procedures established by Moura (2007) in previous monitoring campaigns. The climatic factors of temperature, humidity, and luminosity were measured using a thermohygrometer (EQUITHERM-TH-439) and luxmeter (UV-A MAGNAFLUX). These factors were measured on all animal monitoring days in all campaigns, at two different times (8:00 h and 16:00 h). The measurements were always carried out at the same point, next to the feeder, from May 2015 to May 2016.
The success of reintroduction was evaluated according to the recommendations of White Jr. et al. (2012), where the survival of reintroduced animals is evaluated after one year and should be greater than 50 %, with the reproduction of the reintroduced animals.
Some data related to abiotic factors were not collected on the morning of the first day of each monitoring campaign due to the arrival time at the field. Thus, the missing values were imputed with plausible data values using the mice package. This package provides a method for handling missing data, as the function creates multiple imputations (substitute values) for multivariate missing data. The algorithm can impute combinations of continuous categorical, binary, unordered categorical, and ordered categorical data (Van Buuren et al., 2019).
Data analysis: The values of each evaluated health parameter, the ratio between males and females, and the number of animals sighted by monitoring campaign were tabulated and analyzed through descriptive statistics (mean ± standard deviation) using GraphPad Prism® version 5 software. The completed data table (through the data imputation described above) was used to perform principal component analysis (PCA) and verify how the abiotic data relate to the frequency of visits to the feeder.
The paired t-test was used to verify possible differences between the periods (morning/afternoon) considering the variables 'frequency of visits', 'temperature', 'humidity', and 'luminosity'. The data were distributed normally only for temperature (afternoon) and humidity (morning and afternoon). However, the t-test is robust for non-normality, considering that there are no outliers. The analyses were performed in R software (R Core Team, 2020).
Results
For the preparation of the animals selected for the reintroduction project, fecal samples of all 59 Amazonas spp., which were in the same enclosure at CETAS, were evaluated for the presence of gastrointestinal parasites.
To prepare the animals that were selected for the reintroduction project, fecal samples from all 59 Amazonas spp. in CETAS were evaluated for the presence of gastrointestinal parasites. Heterakis spp. and Eimeira spp. were identified (Table 1). After treatment, a new evaluation was carried out and no parasites were found in the collected samples.
Table 1 Percentage of samples contaminated by the direct, Willis, and sedimented methods in fecal samples of Amazonas spp. from CETAS - Vitória da Conquista, Bahia, before treatment.
| Endoparasites | Direct method (%) | Willis method (%) | Sedimented method (%) |
| Heterakis spp. | 25.29 | 29.86 | 40.23 |
| Eimeira spp. | 9.20 | 14.94 | 17.79 |
| Eimeira spp. / Heterakis spp | 0 | 0 | 1.15 |
The species of Amazonas spp. in the CETAS at the start of the study were (I) 48 Amazona aestiva, (II) 08 Amazona amazonica, (III) 02 Amazona vinacea, and (VI) 01 Amazona rhodocorytha. Only animals of the species Amazona aestiva (81.3 %) continued to be evaluated and prepared for a group to be reintroduced. This species was selected because there were enough specimens to form a more robust group and because it has already been reported in the previously selected reintroduction area.
In the flight capacity evaluation of specimens of A. aestiva, 72.9 % scored three or four, which is compatible with reintroduction. The rest of the animals (27.1 %) exhibited some alteration that compromised their flight capacity, mainly cut wing feathers, and were, therefore, not prepared for reintroduction. Of the 35 remaining animals, two were removed for exhibiting high animal-human interaction. Therefore, 33 animals had compatible flight capacity for release and low interaction with unknown humans since they did not allow the approach of people.
Molecular sexing revealed a proportion of 69.69 % (23/33) of males and 30.30 % (10/33) of females. For the leukocyte evaluation, all animals had values within the expected parameters for the species (Table 2). The clinical evaluation did not identify any signs that indicated the need to run complementary tests. Thus, all 33 animals previously selected continued in the enclosure with environmental and food enrichment to later be sent to the release area.
Table 2 Leukocyte values obtained for all samples of male and female individuals of the species Amazona aestiva pre-selected for participation in the reintroduction project.
| Hematological parameters | Mean and standard deviation (x 109 /l) | Minimum-Maximum (x 109 /l) | Mean and standard deviation (x 109 /l) | Minimum-Maximum (x 109 /l) |
| Males | Females | |||
| Total Value of Leukocytes | 8.843 ± 2.486 | 2.800-14.200 | 8.363 ± 3.625 | 0.196-12.000 |
| Heterophil | 4.690 ± 1.476 | 1.876-7.154 | 4.201 ± 2.072 | 0.196-7.068 |
| Lymphocyte | 3.486 ± 1.444 | 0.728-6.390 | 3.386 ± 1.794 | 0.196-6.000 |
| Monocyte | 0.466 ± 1.122 | 0.094-5.600 | 0.414 ± 0.374 | 0.120-1.298 |
| Eosinophil | 0.220 ± 147.73 | 0.062-0.612 | 0.234 ± 0.173 | 0.072-0.570 |
| Basophil | 0.207 ± 0.187 | 0.056-0.612 | 0.236 ± 0.197 | 0.084-0.570 |
| Total Value of thrombocytes | 1.0733 ± 2.600 | 5.600-14.700 | 10.659 ± 4.960 | 0.196-16.100 |
The avian nursery was opened at 11:30 h and the first animal left at 13:32 h, soon after which other animals accompanied the first animal to perch and vocalize on nearby trees. On the first day, 78.78 % of the animals left the avian nursery, however, 18.18 % of those that left returned to spend the night inside the avian nursery. The doors of the avian nursery were opened in the morning and closed in the evening every day, for a month. In this period, only one female animal did not leave the avian nursery at any time and was returned to CETAS with the withdrawal of the avian nursery. Thus, 32 animals remained in the release area and were monitored for one year in campaigns comprising 340 h of sampling effort and 204 h of bird watching (Fig. 1).
Fig. 1 Map of the State of Bahia. Highlighted in red for the municipality of Condeúba, the site selected to receive individuals of the Amazona aestiva species. Individual of the species foraging in a feeder at the release site.
The fidelity of the animals in the area, using the feeder or the areas surrounding the release site, allowed the identification of 50 % (16/32) of the animals after one year of release (Fig. 2). During monitoring, the reintroduced animals fed on 10 native plant species in the area (Table 3).
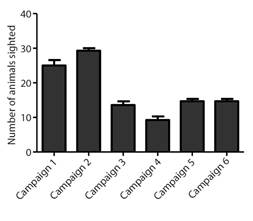
Fig. 2 Demographic census of released parrots that were visiting the feeder or present in the remediations by monitoring campaign. The campaigns occurred as follows: Campaign 1-May 2015 (18 monitoring days), Campaign 2-June 2015 (seven monitoring days), Campaign 3-September 2015 (seven monitoring days), Campaign 4-December 2015 (five monitoring days), Campaign 5-March 2016 (three monitoring days) and Campaign 6-May 2016 (three monitoring days).
Table 3 Record of supplementary food offered at the feeder or by active search of the 32 individuals of Amazona aestiva after reintroduction in Condeuba, Bahia.
| Supplemented foods | Active search foods | |||
| Scientific name | Popular name | Scientific name | Popular name | |
| Musa sapientum | Banana | Plinia cauliflora | Jabuticaba | |
| Citrullus lanatus | Melancia | Citrullus lanatus | Melancia | |
| Carica papaya | Mamão | Eugenia uniflora | Pitanga | |
| Mangifera indica | Manga | Spondias purpurea | Seriguela | |
| Uncaria sp | Unha-de-gato | Morus sp. | Amora | |
| Helianthus annuus | sunflower | Copaifera langsdorffii | Copaiba | |
| Mangifera indica | Manga | |||
| Uncaria sp. | Unha-de-gato | |||
| Passiflora cincinnata | Maracujá do mato | |||
| Psidium guajava | Goiaba | |||
During the monitoring visits to the rural owners near the release site, the team was informed that a resident had trapped a project animal in his home. This animal was rescued and exhibited cut wing feathers, so it was returned to CETAS, and environmental education during the visits was intensified. In addition, community members reported the presence of wildlife hunters in the area (mainly deer and armadillo hunters) and stated that with the constant presence of the monitoring team, the sounds coming from firearms used by these people had decreased, suggesting that they were hunting less frequently.
The formation of five pairs and two trios of animals was identified. From these pairs, we identified the occupation of two artificial nests and one natural nest (occupied by a trio). Three eggs were recorded in only one artificial nest. However, the contents of the nest were predated by a toucan. An individual of Ramphastos toco, Statius Muller, 1776 (toco-toucan), a species native to the area, was seen entering the nest, which indicates that it fed on the parrot eggs (Fig. 3). Two other artificial nests were occupied by pairs of passerines Troglodytes musculus (Naumann, 1823).

Fig. 3 Photographic records of mating monitoring. A. pair of parrots (Ring-CETAS/UFBA 031 and CETAS/UFBA 024) mating; B. eggs in the artificial nest; C. eggs predated by Ramphastos toco.
The PCA results indicate that the two main axes explained 74.17 % of the data variation. Axis one explained 54.91 % of the data variation. Humidity was more associated with the morning period, while temperature and luminosity most influenced the frequency of afternoon visits (Fig. 4).
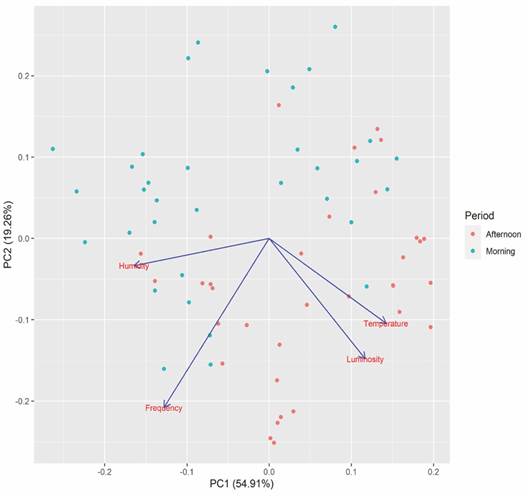
Fig. 4 Principal component analysis (PCA) to evaluate the influence of abiotic factors (temperature, humidity, and luminosity) on animal visits to the feeder in Condeuba, Bahia.
There was a difference between the morning/afternoon periods for the variables: temperature (t = -12.452, df = 33, P < 0.05), humidity (t = 4.626, df = 33, P < 0.05), and luminosity (t = -2.0803, df = 33, P < 0.05). However, this did not influence the frequency of visits between the two periods (t = -1.8686, df = 33, P = 0.07) (Fig. 5).
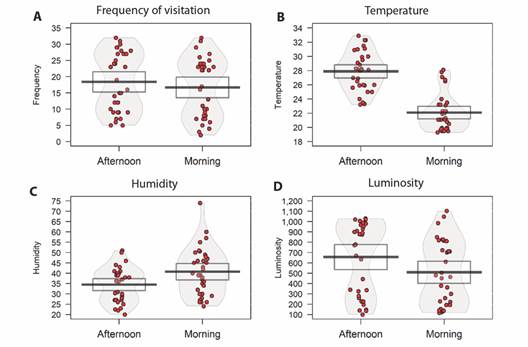
Fig. 5 Evaluation between morning/afternoon periods. In A. frequency of visits to the feeder, in B. temperature differences, in C. humidity, and in D. luminosity. The red dots are the raw data, the horizontal black line represents the mean, bean is the density, and the rectangle is the confidence interval.
Discussion
Reintroduction enables the reestablishment of populations of different parrot species bred for this purpose (Brightsmith et al., 2005; Earnhardt et al., 2014; Smales et al., 2000) or confiscated from illegal trafficking (Sanz & Grajal, 1998; Silva et al., 2021). Therefore, it is an important procedure to enable the destination of psittacines rescued from illegal wildlife trafficking.
Amazona aestiva was the most common parrot species in the CETAS of Vitória da Conquista, Bahia, with 81.3 %. This species is the tenth most frequent among the birds that entered the CETAS of Bahia between 2009 to 2019, with a total of 1 429 specimens (dos Santos, 2021). These figures reveal the complexity of the management and destination of these animals. The most frequently observed problem was the inability to fly due to cut wing feathers that prevent the animals from escaping illegal captivity. Cut wing feathers have also been identified in other rehabilitation centers for wild birds in Brazil (Fitorra et al., 2021). Moreover, poor feather quality was one of the physical abnormalities observed in 36 % of 122 illegally trafficked parrots in Costa Rica (Mora-Chavarría et al., 2017) demonstrating the impact of this problem on reintroduction projects.
In our study, after selecting the group of animals suitable for reintroduction, a bias was observed in the sex ratio of the animals, with a higher number of males (69.69 %). Efforts to maintain a reintroduced bird population should consider the sex ratio of the released animals since the release of both sexes improves long-term population consolidation (Lambertucci et al., 2013). In contrast, the presence of more males tends to increase the probability of extinction of a group (Donald, 2007). Therefore, the release of females should be a priority in future reintroductions in this group.
Health assessments greatly minimize the risks of releasing infected animals (Deem et al., 2008; Jacobson et al., 1999). However, there is no uniformity in the types of tests performed in the different animal preparation protocols. Efforts are needed to determine minimum suggested procedures for the health screening of candidates for reintroduction projects, especially when the high costs of testing hinder the achievement of these projects (Saidenberg et al., 2015). Hematological blood and parasitological of feces parameters are complementary, low-cost tests used in the routine monitoring of animal health (del Pilar-Lanzarot et al., 2001; Karesh et al., 1997; Melo et al., 2019). In the present study, these analyses determined the necessary therapeutic interventions to be performed in the group before they were reintroduced.
The return to the natural habitat after a period of captivity can sometimes be a stressor and reveal new challenges for individuals (Parker et al., 2012). Thus, more attentive care is required in the first stages of reintroduction for the adaptation period in the selected environment. This stage consisted of a period of recognition of the new environment while still in captivity, as recommended by Jones & Merton (2012) and tested for A. aestiva by Silva et al. (2021). The next stage was to open the nurseries using the soft release method (Scott & Carpenter, 1987). Maintaining access to the aviaries even after the animals left proved critical for the parrots to gain confidence in the new area, as it was observed that the parrots returned to spend the night inside the nurseries during the first days of freedom or some parrots did not leave the aviary at any time of the day, despite having gone through all the stages of preparation. The return of the released animals to the aviary was also monitored and identified in other reintroduction projects of psittacids (Silva et al., 2021; Vilarta et al., 2021).
To evaluate reintroduction success in parrots, White Jr. et al. (2012) proposed the criterion of more than 50 % survival of animals released after one year. In the present study, at least 50 % of the released animals that remained in the monitored area survived. Although one bird was captured by humans, no deaths or predation of adults by other animals was recorded. We believe that the other animals dispersed beyond the monitored areas. Given the uncertain fate of some individuals that leave the monitored areas, it is impossible to attest to the level of success of some reintroductions of psittacids (Snyder et al., 1994; Vilarta et al., 2021). Despite the impossibility of evaluating success, dispersal can reveal a positive effect since the population may have occupied new areas (Vilarta et al., 2021).
Prolonged periods of feeder use with supplementary feeding encourage the animals to remain loyal to the release area (Brightsmith et al., 2005; Vilarta, 2021) and facilitates post-release data collection (Tollington et al., 2013). The feeders used in the present study contributed to monitoring by providing the sighting of at least half of the reintroduced animals visiting the feeders or adjacent areas after one year. Supplementary feeding, when not properly monitored, can be counterproductive and cause unwanted effects such as the spread of diseases, nutritional imbalances, or behavioral changes, as in the case of increased fighting and increased predation pressure (Robb et al., 2008; White Jr., 2012), facts not observed in the present study.
Supplementary feeding improves the reproductive performance of birds (Pearson & Husby, 2021) and reproduction is the second criterion presented by White Jr. et al. (2012) to evaluate the success of reintroduction. The frequent monitoring of the reintroduced animals allowed the observation and identification of breeding pairs, mating, and egg-laying in the first year of the study. The provision of artificial nests in reintroduction areas also favors reproduction since it offers immediate nesting opportunities (Tollington et al., 2013; Vilarta et al., 2021; White Jr. et al., 2005). However, the recorded eggs were predated by an individual of Ramphastos toco, which is already known as a potential predator of psittacine nests (Oren & Novaes, 1986; Vilarta et al., 2021). Although the nests used in this study were used by pairs of A. aestiva, some nests were occupied by non-target species. Therefore, the effectiveness of artificial nests should be evaluated regularly to ensure and adapt the conditions to favor the target species (Gautschi et al., 2022).
The evaluation of the impact of abiotic factors on the visits to feeders in the morning and afternoon showed that, despite the difference between the periods for the variables: temperature, humidity, and luminosity, these factors did not influence the animals in this regard. However, the variable humidity best explains morning visits, while the variables temperature and luminosity had the greatest influence on visits in the afternoon. Changes in local climatic conditions lead to physiological adjustments in the metabolism of these animals. Birds exhibit adaptive physiological responses when faced with climate change (Noakes et al., 2016). Environmental temperature and humidity were correlated with duration of daily activity in Amazona amazonica and herons (Hirundo fluvicola and Ardea insignis), where the duration of daily activity was positively correlated with morning and evening temperatures and negatively correlated with sunrise time and morning and evening humidity (Khandu et al., 2022; Moura et al., 2012; Singh et al., 2015).
The results of these past studies may help to understand the pattern observed in the present study, where on wetter days the animals start their activities later, which may influence more visits to feeders in the morning. On warmer days, and with more light, the birds start their foraging activities in more distant areas earlier and only in the afternoon visit the feeders when returning to maintenance areas.
This study confirms the reintroduction of A. aestiva as a viable possibility for the destination of animals seized from wildlife trafficking and the importance of monitoring for at least one year to evaluate and ensure successful reintroduction. Furthermore, food supplementation and the installation of artificial nests in the release area favor monitoring and enable reproduction. We consider that these activities are necessary to achieve the objective of reintroduction projects, which is the establishment of viable populations and the consequent increase in overall chances of survival and conservation of the studied species.
Moreover, we consider that the reintroduction presented here is on the threshold of classification as successful since it was possible to identify the survival of at least 50 % of the animals after one year of release and record reproduction events. In addition, the study presents environmental clues as to how abiotic factors may influence daily behavioral decision-making related to the use of supplementary feeding in reintroduced parrots. We suggest the evaluation of the release of new groups of animals, preferably formed by females, for population reinvigoration. Moreover, further studies can shed valuable light on how abiotic factors can influence the behavioral dynamics of animals reintroduced during climatic seasons.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 


