Introduction
Ecological interactions can have positive, negative, or neutral effects for the species involved in them, and they play a crucial role in the structure and organization of communities (Bertness & Callaway, 1994; Perea et al., 2013). In the dispersal cycle of plants, there are positive interactions with animals such as seed dispersal, and negative interactions such as seed predation (Howe, 1986; Howe & Smallwood, 1982; Janzen, 1971b). These interactions can affect the plant and animal populations involved; on the one hand, they play a role in maintaining the seed bank and, therefore, in seed distribution in the environment, so that the interactions have impact the recruitment of plants and thus affect their fitness. While on the other, animals depend on the resource provided by plants for their survival (Howe & Smallwood, 1982; Janzen, 1971b; Louda, 1989). These interactions occur with very high frequency, so their effects have consequences on plant demography and genetic diversity and are critical in the maintenance and plant diversity (Calvino-Cancela, 2007; Howe & Smallwood, 1982; Janzen, 1971b; Jordano & Godoy, 2000; Wang & Smith, 2002).
Animals, as small invertebrates to large mammals, are agents involved in seed removal (Howe & Smallwood, 1982;. Janzen, 1971b; Martínez-Orea et al., 2009). Rodents are considered seed dispersers (Acevedo-Quintero & Zamora-Abrego, 2016; Ouden et al., 2005; Sunyer et al., 2013; Xiao et al., 2006), and seed predators (DeMattia et al., 2006; Galetti et al., 2015a; Ibáñez & Soriano, 2005; Janzen, 1971b; Traveset et al., 2009). Rodents are important in communities; by excavating and building their burrows, they provide benefits to ecosystems, such as water infiltration, improved soil texture, and changing the level of available nutrients, making soils more heterogeneous (Ewacha et al., 2016; Reichman & Seabloom, 2002; Zhang et al., 2003), increasing landscape variability, and maintenance of species richness in changing environments (Brown et al., 2001; Davidson & Lightfoot, 2008; Reichman & Seabloom, 2002; Valkó et al., 2021; Zhang et al., 2003).
However, the environment has a problem around the world, which is the high social importance (Blackie et al., 2014), as a land-use change that caused habitat fragmentation and altered the original vegetation structure (Emer et al., 2018; Haddad et al., 2015). Approximately 90 % of the tropical deciduous forest (TDF) in the world has been altered by agriculture or ranching (Banda et al., 2016), which increase rodent densities, since these organisms obtain their food more efficiently in farmlands (Castillo-Castillo & González-Romero, 2010; Galetti et al., 2015a), where they can be considered pests (Elias & Valencia, 1984; Villar-González, 2000). In México, for example, crops including corn, sorghum, rice, beans, sugarcane, coconut, and squash are affected by rodents (Bello & Hidalgo, 2009; Brooks & Fiedler, 2001; Panti-May et al., 2017; Villar-González, 2000). The land-use change causes species loss due to migrations to vegetation patches or agricultural areas and even the local extinction of native species. In addition, this species loss induces a decrease in ecological functions, including interactions between plants and animals (Bolger et al. , 1997; Galetti et al., 2015b; Marjakangas et al., 2020).
In Western México, Notocitellus adocetus is a terrestrial squirrel, an endemic rodent species (Flores-Alta et al., 2019; Valdés & Ceballos, 2014), considered a pest by people who refer that squirrels cause damage to their crops; for this reason, they kill individuals of this species. This human activity can change community structure and ecological functions derived from land-use change; therefore, it is essential to know the role of the N. adocetus in the environment to determine conservation strategies for this species. So, the question for this work was, what role does N. adocetus play in seed removal on three plant species? Then the aim of the present study was to evaluate the removal by N. adocetus of C. alata, R. capitata, and Z. mays seeds to determine whether this squirrel species participates in seed dispersal and/or seed predation. Also, evaluate the rodents impact on these plant species to know the importance of this species on the environment.
Materials and methods
Species: Notocitellus adocetus belongs to the Sciuridae family, has terrestrial habits and is known locally as the ''cuinique'', it is commonly referred to as the tropical ground squirrel and is endemic to Western México (Flores-Alta et al., 2019; Valdez, 2003).
The three plant species studied were chosen because they are the most consumed by N. adocetus in the locality of Cuambio, Guerrero (Flores-Alta et al., 2019). Also, in TDF only these species had fruit during the study. The seed of Z. mays is an important seed consumed by humans, to which N. adocetus causes economic losses, for this reason the people kill them.
Crescentia alata and R. capitata are wild TDF species, whose fruit production occurs during the dry season. Zea mays is the most cultivated species in the region (Duque, 2016 personal communication) and produce fruit at the end of the rainy season.
Crescentia alata (Bignoniaceae) (Cirián/Mexican Calabash) is a tree from 6.96 ± 0.92 height (Table 1), has an indehiscent fleshy fruit measuring 7 to 15 cm diameter, with a hard shell, has seeds from 0.6 to 1 cm long (Briones-Salas et al., 2006; CONABIO, 2020; Flores-Alta, 2018; Janzen & Martin, 1982). The fruits of this species are consumed by rodents (Liomys salvi) in Costa Rica (Janzen, 1982). Randia capitata (Rubiaceae) (Tecuche or Cruceta) is a tree from 3.67 ± 0.15 m height (Table 1), has indehiscent globose berry fruits measuring 5 cm in diameter, with a hard shell (Felger et al., 2012; Flores-Alta, 2018), has seeds from 0.79 ± 0.03 cm long and 0.63 ± 0.2 cm wide (Obs. pers.). Zea mays (Poaceae) (corn) is an herb with caryopsis-type fruits that together form an ear; the seed size measure 0.55 to 0.95 cm long and 0.3 to 0.7 cm wide (Espinosa-García & Sarukhán, 1997; Quiroz, 2019).
Table 1 Number and size of individuals of C. alata and R. capitata in tropical dry forest plots.
| Species | Number of individuals | Height (m) | DBH (cm) | Coverage individual (m2) | Coverage (m2) | Coverage (%) |
| C. alata | 0.33 ± 0.16 | 6.96 ± 0.92 | 51.82 ± 92.61 | 49.75 ± 27.01 | 83.13 ± 22.93 | 2.77 |
| R. capitata | 11.66 ± 2.56 | 3.67 ± 0.15 | 5.39 ± 0.52 | 4.84 ± 0.89 | 262.16 ± 0.62 | 8.73 |
DBH = Diameter at breast height.
Study area: The study site is in the Northwest of the state of Guerrero, in the municipality of Zirándaro de Los Chávez in the locality of Cuambio, at coordinates (18°26'28.54''-18º26'06.63'' N & 101°01'21.68''-100º59'39.56'' W), between 209 and 324 m.a.s.l. (Flores-Alta et al., 2019). The mean annual temperature is 28.4 °C, and the mean annual rainfall is 977.2 mm, with a dry season lasting eight months (CONAGUA, 2020). The vegetation type is TDF, with areas that have suffered land-use change on the alluvial terraces. Some sites are used to farm seasonal and irrigation-based crops including corn, sorghum, sesame, and to a lesser extent, mango, watermelon, plum, pumpkin, cucumber, tomato, chili, hibiscus, and beans. In the same way, there are areas dedicated to cattle grazing (Mendoza, in prep.). The extension of the study area in the TDF was 6.45 ha, while in the corn crops was 7.45 ha, and the distance between the two areas was 1.9 km.
For each plant species, we carried out one sampling period during each sampling month, which were selected based on when fruits are produced. For Z. mays, this was in September and October 2016, for C. alata in January, March, and May 2017, and in January and March 2017 for R. capitata. Each sampling lasted three days at two sites. For each of the plant species, we made direct observations at fixed points daily (Altmann, 1974) during the peak activity periods of N. adocetus (9:00-14:00 h) (Flores-Alta et al., 2019). Three observers remained still at different points at a distance of ten to 15 m from the focal plants so as not to disturb the activity of the squirrels and observed their behavior using 10X42 binoculars (Bushnell and Alpen). Each observer attended from one to three focal individuals of C. alata (1.32 ± 0.09 individuals), with 14 individuals observed in total, for R. capitata we observed five to nine individuals (6 ± 1.77 individuals), with 24 individuals observed in total, and for Z. mays for each observer was attended from seven to ten individuals with 35 individuals observed in total (8.3 ± 0.88 individuals).
The total observation time for each species was 32.25 h for C. alata, 50.06 h for R. capitata, and 49.66 h for Z. mays. The lower total observation time of C. alata is due to the lower number of individuals (Table 1) in the study area.
To complement the foraging observations, we placed three digital camera traps (Bushnell Trophy cam HD, Essential E2) near three focal individuals of each of the plant species for three consecutive days (Trolliet et al., 2014). The cameras were focused on open fruits located on the ground in the case of C. alata and R. capitata, while in the case of Z. mays, we placed fruits on the ground. The cameras were programmed to film 60 s videos, which were reviewed in the laboratory. During the foraging observations and videos, we noted the part of the fruit consumed (pulp and/or seeds), stage of development (ripe or immature), number of feeding observations recorded, number of seeds consumed, number of individuals of N. adocetus feeding per event, time foraging (Contreras-González & Arizmendi, 2014), foraging behavior (consumption of seeds in situ or storage in cheek pouches), as well as their behavior and movements after feeding (e.g. to their burrows or places with better visibility, such as rocks).
To quantify the number of seeds that N. adocetus removes and determine its impact on the three plant species studied, we quantified the percentage of seed removal. For this, we placed six fine sand traps (FST) per species per sample close to the parent plant (1.5 m distance), with a minimum of 15 m between traps. We set the traps before the squirrel's activity period in the morning, and we checked them at the end of this activity for two consecutive days. The trapping method consisted of clearing a 1m2 area of litter and vegetation for each trap and placing a layer of fine sand so that the squirrels would leave tracks in the sand when removing the seeds (Giraldo & Moreno, 2011). We put 40 seeds previously obtained from mature fruits at the center of each FST. For FST containing N. adocetus tracks, we quantified the number of seeds removed for each plant species and expressed these data as a percentage. We identified N. adocetus tracks by comparing tracks left in the FST to the tracks of Mexican Ground Squirrel (Spermophilus mexicanus) and Rock Squirrel (S. variegatus) in the Mexican wild mammal tracking guide (Aranda, 2012; N. adocetus are not described in the guide). There is little chance of misidentifying tracks as belonging to N. adocetus, since no similar squirrels are distributed in the area.
Fruit abundance and damage: Because the abundance of resources available in the environment influences seed removal (Izhaki, 2002; Ortiz-Pulido et al., 2007; Wästljun, 1989), we quantified the resources available for N. adocetus. During each sampling in the TDF, we randomly placed three 50 x 20 m plots in which we quantified the number of individuals and the number of fruits per individual of each of the two plant study species. When fruits were too numerous to efficiently count directly, we estimated the number of fruits by multiplying the average fruit count from three branches by the total number of branches per individual (Chapman et al., 1992; Contreras-González et al., 2009). In addition, to know the height, coverage, and space occupied by the individuals of C. alata and R. capitata, we measured the diameter at breast height (DBH), the height of the individuals, and the coverage for each individual using the ellipse formula to estimate the percentage that each species occupies in the sampled space, since these variables influence fruit production (Chapman et al., 1992). In the case of Z. mays, we reduced the plot size to 5 x 5 m due to the high density of plants. We quantified the total number of individuals and the number of fruits per individual.
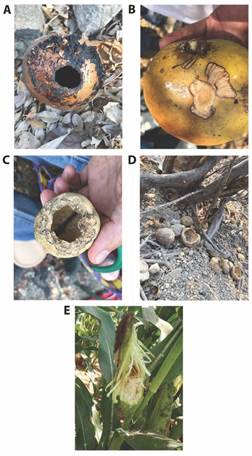
We also quantified the number of fruits with damage caused by N. adocetus (bites, partially opened fruits, or partially consumed fruits; Fig. 1), identifying the source of damage with the help of a person from the Cuambio locality who has observed squirrel activity for many years. We estimate the percentage of damage by dividing the number of damaged fruits by the total fruits in the plot.
We applied Shapiro-Wilk normality tests to all the data obtained to determine whether the data conformed to parametric assumptions (Zuur et al., 2009). Because we did not observe N. adocetus feeding on R. capitata in direct observations or camera trap videos, analyses of some data included only C. alata and Z. mays.
We applied a Wilcoxon test to determine whether the number of squirrels feeding on the fruits differed between C. alata and Z. mays. We calculated the rate of seed removal to evaluate the intensity with which a squirrel consumes the seeds from C. alata and Z. mays by dividing the number of seeds consumed by the time that squirrels spent foraging. We used a generalized linear model (GLM) test with a Poisson error distribution to determine whether the rate of seed removal of N. adocetus differed between these species (Zuur et al., 2009).
To evaluate the differences in the percentage of seeds removed in the FST among the three species studied in the different months sampled, we used a generalized linear mixed model (GLMM) with a Poisson distribution (Zuur et al., 2009), with percentage of seed removed as the dependent variable, and time and species as independent variables. We analyzed the wild TDF species separately from the corn crops because human management influences fruit production in corn crops (Nadal, 1999). For this analysis, we used a linear mixed model (LMM) for the TDF species, with fruit abundance as the dependent variable, and time and species as independent variables and a GLM with a Poisson distribution for the corn crops (Zuur et al., 2009), with crop size as dependent variable, and time as independent variable. In addition, due to pseudoreplication, we applied a GLMM analysis with a Poisson distribution to examine differences in the percentage of damaged fruits between the studied species (Zuur et al., 2009).
Results
We found that N. adocetus feeds on the pulp and seeds of ripe and immature fruits of C. alata and Z. mays (Table 2). We did not record squirrels feeding on R. capitata, though we did observe fruits of R. capitata and C. alata with marks from N. adocetus (Fig 1A, Fig. 1B, Fig. 1C, Fig. 1D). Also, we observed individuals of N. adocetus opening fruits of C. alata by making a hole in the hard shell, from which they extracted pulp and seeds. Squirrels fed on seeds of C. alata and Z. mays in situ, taking seeds with their hands, opening them with their teeth and consuming the embryo, leaving only the seed coat. Some seeds were carried in the cheek pouches to their burrows or to later feed in areas with greater visibility (e.g., on rocks, protruding roots, or dry logs). We observed 1.14 (± 0.06) individuals feeding on 6.47 (± 1.15) seeds of C. alata per visit, while for Z. mays we observed 1.02 (± 0.02) individuals feeding (Wilcoxon = 381, P > 0.05). Squirrels removed more C. alata (removal rate) (13.60 ± 0.24 seeds / minute) seeds per minute than Z. mays seeds (10.42 ± 0.84 seeds / minute) (X2 = 2.55, d.f. = 22, P < 0.05; Table 2).
Table 2 Species eaten by N. adocetus in TDF and corn crops.
| Species | Part eaten and stage of ripenessa | FOR | SF | FT (min) | CR (seed/min) | RFST % | Month |
| C. alata | rp, pl, se | 103 | 1.14 ± 0.06 | 6.47 ± 1.15 | 13.06 ± 0.24 | 29.5 ± 6.15 | Jan, Mar, May 2017 |
| R. capitata | rp, pl, se | 0 | 0 | UN | UN | 23.17 ± 8.41 | Jan, Mar 2017 |
| Z. mays | rp, unrp, dr, pl, se | 6 | 1.02 ± 0.02 | UN | 10.42 ± 0.84 | 43.43 ± 4.88 | Sep, Oct 2016 |
In part eaten and stage of ripeness: rp = ripe, unrp = unripe, dr = dry, pl = pulp, se = seed; FOR = number of feeding observations records; SF = number of squirrels feeding per event; FT = time foraging per event observed; CR= consumption rate; RFST = percentage seed removal in fine sand traps; UN = unidentified).
In the FST, N. adocetus removed seeds from all three study species. The highest seed removal was from Z. mays (43.43 ± 4.88 %; X2= 14.89, d.f. = 122, P < 0.05), followed by C. alata (29.5 ± 6.15 %), and finally R. capitata (23.17 ± 8.41 %) (Table 2).
In terms of resource abundance, in the TDF plots C. alata had higher height and DBH and more coverage than R. capitata (Table 2). However, in the sampled area, C. alata occupied a lower percentage of the area than R. capitata (2.77 % and 8.73 %, respectively) and there were fewer individuals of C. alata than R. capitata (0.33 ± 0.16 and 11.66 ± 2.56 individuals respectively (Table 2). For C. alata, we found a larger number of fruits per hectare than for R. capitata. C. alata had higher fruit abundance in March than in January and May, while R. capitata had similar fruit abundance between January and March, and we did not record fruits in May (Fig. 2A, LMM = 11.68, d.f. = 74, P < 0.05). In the corn crops we recorded more fruits in September than in October (Fig. 2B, X2 = 6.07, d.f. = 14, P < 0.05).
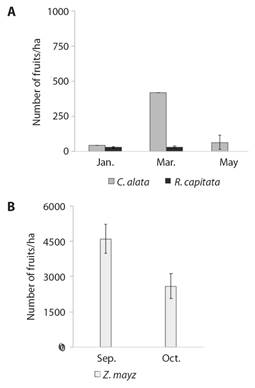
Fig. 2 Abundance of fruits per hectare in study plots in C. alata and R. capitata in tropical dry forest plots A. and in B. Z. mays in plots in corn fields.
Based on the number of available fruits of the studied plant species, we found that the percentage of fruits damaged by N. adocetus was highest for C. alata (38.74 ± 11.92 %), while for R. capitata it was 10.34 % (± 5.75) and for Z. mays was 5.16 % (± 2.55; GLMM= 1.82, d.f. = 74, P > 0.05).
Discussion
Notocitellus adocetus feeds on the pulp and embryo of the seeds of C. alata and Z. mays, which indicating that this rodent is a seed predator (Janzen, 1971b), as is the case of the other squirrels in tropical forests (Notosciurus granatensis, Sciurus variegatoides, S. colliaei y S. ingrami) (Acevedo-Quintero et al., 2018; Henn, et al. 2014; Herrerías-Diego et al., 2008; Janzen, 1971a). In addition, when N. adocetus fed on C. alata, it scared away reptiles and birds, and some members of these groups are considered seed dispersers (Howe & Smallwood, 1982; Valido & Olesen, 2007). However, when squirrels feed on C. alata, the iguana Ctenosaura pectinata scares them away. Members of the Iguanidae family have been described as seed dispersers, increasing the reproductive success of some plants (Traveset, 1990; Vásquez-Contreras & Ariano-Sánchez, 2016), which can influence the seed dispersal from C. alata.
One of the feeding behaviors we observed was that N. adocetus made a hole in the tough outer shell of C. alata through which it extracted the pulp and seeds. This behavior has also been described in the squirrel S. variegatorides in Costa Rica, which can spend up to 15 min making the hole in the fruit, then extracts the pulp in pieces and feeds on the seeds (Janzen, 1982). The characteristics of C. alata and R. capitata fruits, such as a thick, hard covering, make it difficult for other organisms to access the seeds. In addition, when the fruits of C. alata fall to the ground and mature, the seeds can die due to desiccation when not opened (Janzen, 1982). Therefore, N. adocetus, by breaking the fruits of C. alata and R. capitata, plays an important role since it makes the seeds available to seed dispersers, as occurs for the plant species Heteroflorum sclerocarpum (Urrea-Galeano & Andresen, 2018). Similar dynamics have been described for the squirrels S. granatensis, Microsciurus mimulus, and the rat, Proechymys sp., which make the seeds of Oenocarpus bataua available to seed dispersers (Rojas-Robles et al., 2012).
Notocitellus adocetus removed a higher percentage of C. alata seeds in the FST, influencing negatively on this plant species (Vander-Wall et al., 2005). However, this rate of removal is lower than chipmunks Tamias amoenu, which can consume a seed per second (Vander-Wall, 1994). Seed removal by N. adocetus in the TDF and the corn crops is lower compared to other rodent species, since has been described seed removal by rodents for the forest 96 % of seed removal, and in agroforestry systems 76 % (Escobar et al., 2020; Li & Zhang, 2007). This indicates that the effects of N. adocetus on the plant species consumed are likely less severe than other rodent species, which can be useful for conservation programs for this species.
Individuals from N. adocetus feed on fruits and seeds of C. alata and Z. mays in situ. However, some seeds are carried in their cheek pouches to their burrows or to places with better visibility to continue feeding. This behavior could lead to some seeds being dispersed when they are dropped during transport or feeding (Gottfried, 1987). Moreover, outside burrows of N. adocetus, we found empty fruits of R. capitata with holes (Fig. 1D); the same feeding behavior has been reported for this squirrel species when it feeds on H. sclerocarpum (Urrea-Galeano & Andresen, 2018). Squirrels can store seeds temporarily in their burrows, which can even germinate when squirrels fail to find and eat all of the seeds they have cached (Steele et al., 2015; Vander-Wall, 1994; Vander-Wall, 2003; Zong et al., 2010). However, the absence of large seed dispersers due to fragmentation has led to a loss of ecological interactions and an increase in rodent populations, such that seeds are deposited mainly in an aggregate manner (Marjakangas et al., 2020; Rojas-Robles et al., 2012), which may affect plant populations (Hulme, 1998; Hulme, 2002).
We found that Z. mays had the highest abundance of fruits of the three species studied, which influences that more seeds of this species are removed (Elmouttie, 2009; Kelrick et al., 1986). There is a higher density of squirrels in the corn crops (Flores-Alta et al., 2019), which increases the intensity of seed predation (Gharnit et al., 2020; Minor & Koprowski, 2015). This is similar to what occurs with the mouse Peromyscus leucopus, in which seed predation increases with increasing population density (Ostfeld et al., 1997). In addition, when resource abundance is high in a small area, rodents do not have to move long distances to acquire resources, so they are less exposed to predators (Hannon et al., 2006).
Although total seed removal was higher in corn crops than in the forest species, the percent damage in plots of Z. mays was low. Conversely, C. alata had higher percent damage, although the density of squirrels is lower (Flores-Alta et al., 2019). Nevertheless, in sub-deciduous forests, the intensity of seed predation by rodents is less intense than in fragmented sites (Fleury & Galetti, 2006), since fragmented areas do not present the appropriate conditions for the rodents' predators. In addition, squirrels have better visibility to detect predators when they feed in fragmented areas than in forests (Fleury & Galetti, 2006; Hannon et al., 2006; Herrerías-Diego et al., 2008).
Due to the interactions present in the environment, it is necessary to observe the ecological and evolutionary history of the plant species. Unfortunately, there is no information for R. capitata. However, C. alata has been described as having a rare adaptation to survive and avoid seed predation which makes the fruit inaccessible to most animals. Members of the now extinct proboscidean family Gomphotheriidae were proposed as their previous dispersers (Janzen & Martin, 1982). Once the members of this family became extinct, other organisms, such as horses, dispersed the seeds (Janzen, 1982). However, these organisms could not be considered seed dispersers in the study area in the TDF since their distribution is restricted to farmlands and grazing lands and is hardly found in the TDF. So that is necessary to carry out studies from seed dispersal for both species.
We conclude that N. adocetus is a seed predator of the three species studied, which can decrease or regulate the populations of these plant species; however, the intensity of seed predation is not a result of the number of fruits. Nevertheless, the characteristics of sites in TDF, such as the proximity to areas dedicated to farmland and grazing land can influence seed predation, so it would be essential to carry out a study that quantifies the predation of seeds in conserved areas far from disturbed areas.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio