Introduction
Habitat alterations associated with global changes negatively influence ecosystems (Hooper et al., 2012). For instance, in tropical montane areas there is an urgent need to monitor biodiversity (Rakotomalala et al., 2021; Roach et al., 2020). For example, in regions of high diversity and endemism, such as the tropical high Andes, rapid land-use change results in an increase in habitat degradation, and this conversion of natural habitats is linked to a biodiversity loss (Myers et al., 2000). Birds are indicators of ecosystem health, being an integral component of habitats and important to ecosystem services (Hudson et al., 2014; Sekercioglu, 2006). Raptors and scavengers provide important services such as prey control and recycling of carcasses, which controls the spread of diseases (Bregman et al., 2014; Sekercioglu, 2006). Raptors are directly related to ecosystem health and are also considered indicator species because they have different degrees of sensitivity as their presence or absences indicates habitat changes (Butet et al., 2022; Pruscini et al., 2016; Sekercioglu, 2006).
The páramo is a distinctive ecosystem of the high Andes of Northern South America (Neill, 1999). Here, raptors stand out as a community with a high concentration of threatened species, whose populations are in persistent decline due to illegal hunting and habitat loss (Ballejo et al., 2018; Naveda-Rodríguez et al., 2016; Plaza & Lambertucci, 2020). Important threatened species in this community include Vultur gryphus (Andean Condor), Falco peregrinus (Peregrine Falcon), Falco femoralis (Aplomado Falcon) and endemics such as Phalcoboenus carunculatus (Caracara Curiquingue) (Fjeldsa & Krabbe, 1990; Freile & Restall 2018; Freile et al., 2019; Ridgely & Greenfield, 2001). Therefore, to improve monitoring efforts for the high Andean raptor community, formal descriptions of field methods and data analysis of the community assemblage is crucial in order to improve management and conservation plans in the region.
In the páramo landscape of the Southern Andes of Ecuador, preliminary monitoring efforts have focused on intensive searches and ecological modelling at a species scale (i.e., species-by-species manner), notably include threatened species such as V. gryphus (e.g., Astudillo et al., 2011; Astudillo et al., 2016; Naveda et al., 2016). However, no efforts have concentrated on the entire raptor community. Furthermore, the raptor community is characterised by low population densities across large areas (Newton, 1979; Pruscini et al., 2016), resulting in limited efforts to monitor the entire raptor community, as well as complications in data analyses due to low detections, evidencing the need for robust monitoring and analysing protocols. In this context, the present study assesses the high Andean raptor community in the páramo grassland ecosystem of Southern Andes of Ecuador through annual monitoring across transects. Community structure is then explored by means of multivariate analysis (i.e., principal components) to determine the species associations that comprise the community.
Materials and methods
Study area: Our study was carried out the core area of the Macizo del Cajas biosphere reserve in 31 761 ha (3 % of the biosphere reserve) of protected páramos in the province of Azuay, Southern Ecuador (Fig. 1). The study boundaries include the Quimsacocha National Recreation Area and buffer zone (i.e., 10 km radius; 3°3'16'' S & 79°14' 56'' W). The monitoring encompassed around 7 434 ha (i.e., monitoring area influence; Fig. 1). The landscape is characterized by an irregular topography composed of deep, U-shaped valleys, steep slopes, with rivers on the valley floors and rock mounds at mountain tops (Rodríguez et al., 2014). The elevational range in the study area is 3 430 m.a.s.l. to 3 900 m.a.s.l. The monthly average temperature is 5.4 °C with a maximum of 13.8 °C and a minimum of -0.9 °C. Annual rainfall varies from 1 000 mm to 1 250 mm. The highest precipitation occurs between December and May (Campozano et al., 2016; Ochoa-Sánchez et al., 2018). The vegetation is dominated by páramo grassland (90 % of the vegetation cover), an open habitat with herbaceous plants (mainly of the genus Calamagrostis) aggregated in tussocks, and associated with shrubby plants of the genera Chuquiraga, Gynoxys, Monticalia, Valeriana and Loricaria (Baquero et al., 2004; Neill, 1999).
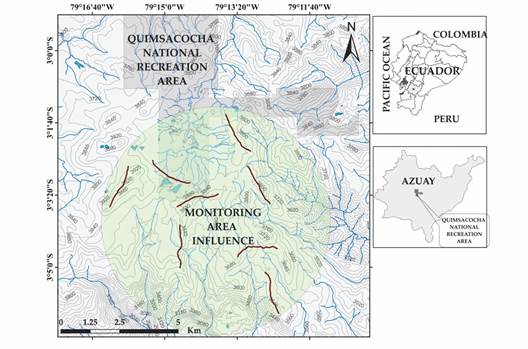
Fig. 1 Map showing the study area and location of eight transects for raptor monitoring in the páramo ecosystem, high Andes of Southern Ecuador. The grey shaded polygon represents the Quimsacocha National Recreation Area. The monitoring area influence is 7 434 ha. The secondary map shows the province of Azuay (grey polygon) in Ecuador.
Bird monitoring: Raptor monitoring followed the protocols described by Astudillo et al., (2011) adapted from Ralph et al., (1996) for the Southern Andes of Ecuador. In total, eight transects of 2 km in length separated by at least 700 m were established. The distance between transects was defined to ensure data independence. All transects were located in wide-open sites to ensure an observation radius of at least 1 km. To avoid double counting, censuses were carried out by two observers who walked each transect at a constant speed and recorded all raptor individuals seen, including those flying overhead. Transects were surveyed only on days with good weather conditions, avoiding periods of rain, fog, and low visibility. Each transect was conducted for three hours once a month from October 2021 to September 2022, with surveys occurring between the hours of 07:00 to 17:00. The total surveying effort was 288 hours of observation. The transects were walked in a random order each month. Taxonomic identification of species follows the classification of the South American Classification Committee (Remsen et al., 2021).
Data analysis: Total abundance was considered as the sum of all individuals per species and per transect (Nur et al., 1999). Community structure was described by principal component analysis (PCA) (Gewers et al., 2021; Huettmann & Diamond, 2001). This technique is one of the most widely used approaches for the analysis and description for biological community data (Vaughan & Ormerod, 2005). The PCA is effective in solving problems associated with different numbers of variables (e.g., species), multicollinearity and small sample sizes (Graham, 2003; Jankowski et al., 2009). Therefore, we used a PCA ordination, based on a correlation matrix of species abundances against sites. The most important components of this ordination were then chosen via the broken-stick method (Jackson, 1993). Sites and species were projected onto a two-dimensional space defined by the chosen components (i.e., biplot), sites closer to each other within the two-dimensional space are considered more similar (Jankowski et al., 2009; Palacio et al., 2020); this graphical visualization of the biological data allows us to make a global description of the variation in the community data (i.e., species and sites).
We used the loadings of PCA to interpret the model (correlation coefficient between the species abundance and the principal component scores), which utilizes the relative contribution of each variable to each component (Palacio et al., 2020). To define the raptor community as a species set, we considered only loadings with values ≥ 0.25 as a conservative threshold (i.e., 75 % of the data is retained) to summarise each component.
In order to identify the species that integrate each raptor community, we explored an additional graphical option. We created scatterplot with PCI and PCII scores for each site versus the abundance of each species per site to visualise the importance of species comprising each community component (i.e., PCI and PCII). Species within each community were identified via establishing cut-off points; these points are limits of a given graph section where changes in the abundance of species can be easily identified (i.e., through an increase or decrease in the abundance of species, and/or the appearance or disappearance of other species) (Ballance et al., 1997; Huettmann & Diamond, 2001). In other words, the scatterplot allows us to visualise a dramatic or evident change in abundance of species associated with PCI and PCII.
Results
In total, 149 individuals were recorded associated with seven species and three families (Table 1). The most abundant species was P. carunculatus (51.7 % of records), followed by Geranoaetus polyosoma (Variable Hawk) (27.5 % of records) and Cathartes aura (Turkey Vulture) (12.7 % of records). The least abundant was V. gryphus (Andean Condor) (4 % of records), F. femoralis (Aplomado Falcon) (2 % of records), Circus Cinereus (Cinereous Harrier) (1.7 % of records) and F. peregrinus (Peregrine Falcon) (< 1 % of records).
Table 1 List of raptor species and their total abundances recorded at eight transects in the páramo grassland ecosystem, high Andes of Southern Ecuador from October 2021 to September 2022.
| Order | Family | Common name | Species | Code | Abundance |
| Cathartiformes | Cathartidae | Turkey Vulture | Cathartes aura | CAAU | 19 (mean = 2.38; ± SD = 1.60) |
| Andean Condor | Vultur gryphus | VUGR | 6 (mean = 0.75; ± SD = 1.16) | ||
| Accipitriformes | Accipitridae | Variable Hawk | Geranoaetus polyosoma | GEPO | 41 (mean = 5.13; ± SD = 4.22) |
| Cinereous Harrier | Circus Cinereus | CICI | 2 (mean = 0.25; ± SD = 0.46) | ||
| Falconiformes | Falconidae | Aplomado Falcon | Falco femoralis | FAFE | 3 (mean = 0.38; ± SD = 0.74) |
| Peregrine Falcon | Falco peregrinus | FAPE | 1 (mean = 0.13; ± SD = 0.35) | ||
| Carunculated Caracara | Phalcoboenus carunculatus | PHCA | 77 (mean = 9.63; ± SD = 9.29) |
Community structure: The first three components of our PCA explain 91 % of the total variance in the occurrence of seven raptor species in eight transects in the páramo grassland ecosystem (Table 2). However, through the broken-stick method, only the first two components were retained (74 %). The first component (PCI = 47.4 %) reflects a gradient of change from lower abundance to increased abundance of P. carunculatus, G. polyosoma, V. gryphus and C. aura; while the second component (PCII = 27 %) reflects a gradient of change from lower to higher abundance of C. cinereus (Cinereous Harrier), F. peregrinus and F. femoralis (Table 2).
Table 2 Loadings of the first three components of the principal component analysis (PCA) for seven raptor species recorded at eight transects in the páramo grassland ecosystem, high Andes of Southern Ecuador.
| Species | PCI (47.4 %) | PCII (27 %) | PCIII (16 %) |
| Turkey Vulture (Cathartes aura) | 0.421 | 0.253 | |
| Andean Condor (Vultur gryphus) | 0.438 | -0.413 | |
| Variable Hawk (Geranoaetus polyosoma) | 0.448 | -0.267 | |
| Cinereous Harrier (Circus cinereus) | 0.683 | ||
| Aplomado Falcon (Falco femoralis) | 0.339 | -0.736 | |
| Peregrine Falcon (Falco peregrinus) | 0.326 | 0.491 | 0.343 |
| Carunculated Caracara (Phalcoboenus carunculatus) | 0.531 |
The first two PCA components were chosen to determine community structure, no loadings < 0.25 are shown (see methods).
The community ordination (2D solution) showed that PCI is represented by transects composed of more abundant or common species (e.g., P. carunculatus and G. polyosoma) and are located towards the right side of the biplot (Fig. 2). The PCII axis is represented by transects that are composed of the less abundant or rare species (e.g., C. cinereus and F. peregrinus) and are located towards the upper side of the biplot (Fig. 2).

Fig. 2 Ordination biplot of the raptor community recorded in eight transects (green circles) in the páramo grassland ecosystem, high Andes of Southern Ecuador. The ordination shows the first two components of the principal component analysis (PCA). The arrows describe the loadings of the species that integrate the raptor community. Blue lines represent the vectors associated with species whose greatest contribution is to the first component of the PCA (PCI) and those in red for the second component of the PCA (PCII). For species codes refer to Table 1.
By visually identification of the species composing the raptor community (Fig. 3), cut-off points were determined for each component. In the community represented by the PCI, the abundance of more common species increases at PCA-derived scores greater than 2.86 (Fig. 3A); while in the community represented by the PCII, the abundance of rare species increases at scores greater than 2 (Fig. 3B); these changes show a gradient of variation in the abundance of the raptor community.
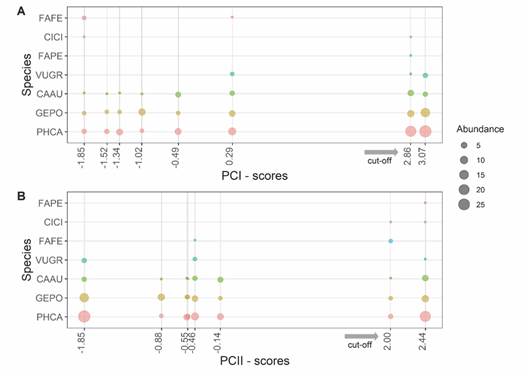
Fig. 3 Scatterplot of abundance by species and their relationship to scores derived from principal component analysis (PCA) for the raptor community surveyed at eight transects in the páramo grassland ecosystem, high Andes of Southern Ecuador. A. corresponds to the community described within the first component of the PCA (PCI) and B. is the second community described within the second component of the PCA (PCII). Grey arrows show cut-off points: > 2.86 for PCI and > 2 for PCII (see methods). For species codes refer to Table 1.
Discussion
The patterns of raptor diversity observed during the surveys are consistent with the general patterns reported for the páramos in the Southern Andes of Ecuador (e.g., Astudillo et al., 2015). Our analysis of the occurrence of seven raptor species in eight transects in the páramo grassland ecosystem of the Macizo del Cajas biosphere reserve revealed two distinct raptor communities. Differences in these two communities as revealed by a PCA are related to species abundance. One community is characterized by a higher abundance of generalist as well as regionally common or uncommon species. However, when the abundance of these common species decreases, the raptor community is characterised by an increase in the abundance of specialist and rare species at the local scale. Competition among species for the limited resources in this ecosystem is probably a potential factor that may explain the variations in the raptor community.
The first community is comprised of species that are more typical of the páramo landscape, particular across the study area. Species associations including G. polyosoma and P. carunculatus are expected, as these raptors are common and widely distributed in páramo ecosystem (Astudillo et al., 2015; Freile & Restall, 2018). However, our results also show that the common species that integrate the community may also be associated with species such as V. gryphus and C. aura. The former species is reported as rare in the páramos due to its low population densities (Freile & Restall, 2018; Naveda-Rodríguez et al., 2016), while the latter is generally rare in the páramo (Astudillo et al., 2015; Olmedo, 2019). On the other hand, V. gryphus and C. aura are both scavengers and their occurrence has been previously linked to the presence of more common species, such as P. carunculatus (Astudillo et al., 2011; Stucchi & Figueroa, 2010). Peisley et al., (2017) and Sekercioglu, (2006) mention that raptors indirectly lead other species to prey or food sources. In this context, in our study area, especially when G. polyosoma and P. carunculatus were sighted together, the presence of V. gryphus tended to be more frequent. Our findings demonstrate that a community dominated by common species may also be integrated by less common species important for conservation (e.g., V. gryphus) and is therefore a characteristic community of the regional páramo.
A second community consists of locally rare species such as C. cinereus, F. femoralis and F. peregrinus. These species forage relatively close to páramo ground level, flying at medium altitude and with a more localized distribution (Freile & Restall, 2018; Ridgely & Greenfield, 2001). Within this context, we consider that competition between species of the first community against. species of the second community could play a fundamental role in determining the structure of this second raptor community (Ballejo et al., 2018; Donázar et al., 2016; Han et al., 2021), where competitive exclusion of certain species may occur when they share a similar role (Sergio & Hiraldo, 2008; Vrezec & Tome, 2004). For example, C. cinereus was observed more frequently when P. carunculatus decreased in abundance at transects, perhaps because both species have similar ground-level foraging habits (Fjeldså & Krabbe, 1990; Freile & Restall, 2018; Ridgely & Greenfield, 2001). However, more studies are required to clarify this relationship. For example, phylogenetic diversity could be employed to understand whether competition is an important factor in species assemblages (e.g., Han et al., 2021).
Our method of characterizing raptor communities through intensive walking transects surveys and principal component analysis proved to be valid for describing and helping to understand páramo raptor community structure. The determination of cut-off points in the analysis is appropriate to interpret and identify changes in the raptor community. Huettmann & Diamond, (2001), for example, mention that this approach can be used when species abundances are very low. Therefore, this approach is especially relevant in habitats where raptors have been little studied due to their low densities and wide distribution ranges such as high Andean ecosystems. In addition, we have shown that this approach represents a more comprehensive monitoring protocol that encompasses the overall community assemblage rather than the more common species-by-species assessment, which fails to take into account possible interactions between species. Furthermore, the approximation of principal components and their cut-off points (based on PCA scores and the relationship with their abundances) can be seen as an alternative for understanding the importance of species even when records are scarce. The páramo regional application of the methods described in this study could help to determine species associations, its turnover and overlap at a landscape scale. It can also be used as a tool for recognising conservation priorities for threatened species.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 


