Introduction
Sea urchins (hereafter referred to as echinoids) comprise an integral component of many shallow benthic marine ecosystems, modifying habitats by grazing algae and seagrasses, creating new habitats through bioerosion of hard substrates, and providing prey for predators (Steneck, 2013). In addition, the pointed spines of echinoids and the burrows echinoids create provide shelter for a variety of invertebrate and vertebrates ectosymbionts, including many species of crustaceans (e.g., Bruce, 1976; Hayes et al., 2016; Ross, 1983) and fishes (e.g., Giglio et al., 2017; Hayes et al., 2019; Karplus, 2014).
Previous studies have demonstrated that several species of crustaceans (Castro, 1978; Hayes et al., 2016; Joseph et al., 1998) and fishes (Giglio et al., 2017; Gould et al., 2014; Hartney & Grorud, 2002; Hayes et al., 2019; Magnus, 1967; Tamura, 1982) prefer to associate with models, live individuals, or species of echinoids with longer or denser spines, presumably to gain protection from predators. Some species of crustaceans (Bruce, 1976; Bruce, 1982; Chace, 1969; Fricke & Hentschel, 1971; Lewis, 1956; Patton et al., 1985) and fishes (Eibl-Eibesfeldt, 1961; Fricke, 1970; Gould et al., 2014; Hartney & Grorud, 2002; Lachner, 1955; Magnus, 1967; Strasburg, 1966; Tamura, 1982) are nearly always associated with long-spined echinoid species; these obligate ectosymbionts often exhibit morphological adaptations such as matching the color of echinoid hosts, possessing dark horizontal lines that are aligned with echinoid spines, or changing color when departing from echinoid hosts. However, many species of crustaceans (e.g., Hayes, 2007; Hayes et al., 2006, Hayes et al., 2016) and fishes (e.g., Giglio et al., 2017; Hayes et al., 2019; Karplus, 2014) associate facultatively with echinoids, sometimes only during the juvenile stage of their life cycle, and lack specialized morphological adaptations for associating with echinoids.
The frequency of ectosymbionts associated with echinoids in the eastern Pacific Ocean has been studied for only two species of echinoids: Centrostephanus coronatus in California (Hartney & Grorud, 2002) and Echinometra vanbrunti in Colombia (Schoppe & Werding, 1996; Vallejo, 2007). In this study we provide data on the frequency of five species of decapod crustaceans (hereafter referred to as decapods) and 14 species of fishes associated with six species of echinoids in shallow water (< 4 m) and deeper water (5-20 m) at Los Cabos, Baja California Sur, Mexico. We evaluate the hypothesis that decapods and fishes associate most frequently with echinoid individuals and species with the longest spines, presumably to reduce the risk of predation.
Materials and methods
Study area: Our study sites were located along the coast between Cabo San Lucas (22°52’36” N & 109°53’46” W) and Playa Palmilla (23°00’34” N & 109°42’55” W), within the Municipality of Los Cabos in Baja California Sur, Mexico, at the southern tip of the Baja California Peninsula. The study area occurs within the Del Cabo biogeographical province (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, 1997) and represents the southwestern border of the Gulf of California. The coastal habitats comprise a mixture of sandy beaches and rocky shores. Scattered patches of stony corals and algae cover subtidal rocks. The marine ecosystems of the area are strongly influenced by seasonal movements of the Inter-Tropical Convergence Zone, which determines the southern limit of the southward-flowing California Current and the northern limit of the northward-flowing Costa Rica Current (Lavín & Marinone, 2003). The marine ecology of the Southern Gulf of California region, which is rich in macrofaunal diversity, is described by Brusca et al. (2005), Brusca (2010), Ganster et al. (2012), Lluch-Cota et al. (2007), and Thomson et al. (2000).
Sampling methods: During 1-6 January 2019 we visually surveyed the decapod crustaceans and fishes associated with echinoids in rocky subtidal areas of the study area. No specimens were collected. Snorkeling equipment was used to survey echinoids in water < 4 m deep at five localities: Playa del Amor (22°52’36” N & 109°53’46” W), Playa las Viudas (22°55’21” N & 109°49’24” W), Playa Santa María (22°55’46” N & 109°48’56” W), Playa el Chileno (22°56’49” N & 109°48’23” W), and Playa Palmilla (23°00’34” N & 109°42’55” W). Scuba equipment was used to survey echinoids in water 5-20 m deep at two localities: Pared Norte (22°52’44” N & 109°53’57” W) and Roca Pelícano (22°52’44” N & 109°53’54” W). To avoid sampling the same echinoids twice, each species was surveyed by a different observer. We surveyed an area of approximately 6.94 ha (measured by the dimensions of areas surveyed, which we plotted on Google Earth; www.google.com/earth). Surveys were conducted only during periods of fair weather when the skies were clear or cloudy and the sea surface was relatively calm.
To obtain data on the densities of shallow-water echinoids, at Playa el Chileno only we counted the number of echinoids of each species in three circular 100 m2 plots for a total of 300 m2. At each study site we carefully inspected each echinoid observed for decapods and fishes. An association was considered to occur whenever a decapod or fish sought shelter within 5 cm of the spines of an echinoid and remained within 5 cm of the spines for at least 10 s when we approached. Decapods and fishes observed > 5 cm from an urchin were not considered to be associated with an urchin. We did not inspect echinoids that were partially hidden within a crevice so that the base of the echinoids could not be adequately observed. We counted the number of individuals of each decapod and fish species associated with each individual echinoid. For the diadematid echinoids Centrostephanus coronatus and Diadema mexicanum, which are the longest spined echinoid species and varied the most in spine length, we used cm markings on the edge of an underwater writing slate held near each echinoid to estimate spine length based on one of three categories: 0-5 cm, 5-10 cm, and > 10 cm. We did not attempt to precisely measure the spines because inserting a ruler within the spines would have disturbed the echinoids and broken off some spines. All data were written on underwater writing slates.
The echinoids, decapods, and fishes were identified directly in the field or photographed and subsequently identified based on field guides (Allen & Robertson, 1994; Bertsch & Aguilar Rosas, 2016; Hickman, 1998; Kerstitch & Bertsch, 2007; Robertson & Allen, 2015) and technical literature (Alvarado et al., 2015). The long-spined diadematid echinoids C. coronatus and D. mexicanum are difficult to distinguish in the field. Centrostephanus coronatus has banded spines in contrast with the uniformly dark spines of D. mexicanum (Bertsch & Aguilar Rosas, 2016; Hickman, 1998), but the spines of juvenile D. mexicanum are often banded (Alvarado et al., 2015) and the spines of some large C. coronatus are nearly black (Pawson & Miller, 1983). Centrostephanus coronatus is best diagnosed by small, purple-tipped, club-shaped spines on the aboral surface, which are difficult to observe while inspecting echinoids in the water, and small spines surrounding the peristome on the oral surface, which requires picking up an individual and turning it over for examination (Alvarado et al., 2015; Bertsch & Aguilar Rosas, 2016; Pawson & Miller, 1983). None of the echinoids, decapods, or fishes were handled or collected. Our taxonomy is based on Solís-Marín et al. (2005) for species and Kroh & Smith (2010) for the sequence of echinoids, De Grave et al. (2009) for decapod crustaceans, and Froese and Pauly (2021) for fishes.
Statistical analysis: The percent frequency of echinoids occupied by decapods and fishes and the mean number of decapods and fishes per echinoid were calculated separately for each echinoid species in shallow water (< 4 m) and deeper water (5-20 m). Because of the difficulty of identification, we combined the data for the diadematids D. mexicanum and C. coronatus in deeper water, where both species were recorded (only one species was recorded in shallow water). A Mann-Whitney U test (z statistic; Zar, 2010) was used to compare spine length of the diadematids between shallow and deeper water. Chi-square analyses of contingency tables (X2 statistic; Zar, 2010) or Fisher exact tests (with exact P value) were calculated to compare the proportions of decapods, fishes, and all ectosymbionts combined associated with different species of echinoids and in different water depth categories. When sample sizes were too small to avoid an expected frequency of < 1, no chi-square analyses were used. Kruskal-Wallis tests (H statistic; Zar, 2010) or Mann-Whitney U tests were used to compare the number of decapods, fishes, and all ectosymbionts combined per echinoid of different water depths in different water depth categories. Spearman rank correlation coefficients (rs statistic; Zar, 2010) were calculated to compare the number of decapods, fishes, and all ectosymbionts combined with spine length categories of the diadematids in different water depth categories and for all data combined.
Results
Echinoid diversity, morphology, and abundance: We visually inspected 1,058 echinoids of six species. The longest-spined were the diadematids D. mexicanum and C. coronatus, with very thin spines up to about 15 cm and 12.5 cm long, respectively. Two species had medium-length spines: the echinometrid Echinometra vanbrunti with thin spines up to about 6 cm long and the cidarid Eucidaris thouarsii with thick spines up to about 5 cm long. Two species had short spines: the toxopneustids Tripneustes depressus with thin spines up to about 2 cm long and Toxopneustes roseus with thin spines up to about 1 cm long.
In shallow water (< 4 m) at Playa el Chileno, E. vanbrunti was the most common species (0.16 ind./m2), followed by D. mexicanum (0.14 ind./m2), T. depressus (0.12 ind./m2), T. roseus (0.02 ind./m2), and E. thouarsii (0.02 ind./m2). No individuals of C. coronatus were observed in shallow water. In deeper water (5-20 m) at Pared Norte and Roca Pelícano, the most common species was D. mexicanum followed by E. thouarsii and C. coronatus. Large individuals of the latter species were distinguished from D. mexicanum by their banded spines, but some unbanded individuals may have been C. coronatus; small individuals of both D. mexicanum and C. coronatus were banded and unidentified. We estimated that 85 % of the larger diadematids in deeper water were D. mexicanum and 15 % were C. coronatus. Spine length of the diadematids averaged shorter in deeper water (mean size class = 1.96, SD = 0.41, N = 95) than in shallow water (mean size class = 2.61, SD = 0.51, N = 370; z = 10.05, P < 0.001). No individuals of T. roseus, T. depressus, or E. vanbrunti were observed in deeper water.
Decapod-echinoid associations: Five species of decapods associated with three species of echinoids (Table 1). The frequency of echinoids hosting decapods in shallow water (< 4 m) differed significantly among the five species of echinoids (X2 = 87.2, d.f. = 4, P < 0.001) and was 5.1 times higher for the longest-spined species, D. mexicanum, than that of any other echinoid species (16.2 % vs 3.2 % for E. thouarsii; Table 1). No decapods were observed associated with the two species of echinoids with the shortest spines, T. roseus and T. depressus (Table 1). The mean number of decapods per echinoid in shallow water was 7.0 times higher for the longest-spined species, D. mexicanum, than that of any other echinoid species (0.21 vs 0.03 for E. thouarsii; H = 271.7, P < 0.001; Table 1). The frequency of echinoids hosting decapods in deeper water did not differ significantly between the diadematids and E. thouarsii (2.1 % vs 3.0 %; Fisher exact P = 1.0; Table 1) and the mean number of decapods per echinoid in deeper water did not differ significantly between the diadematids and E. thouarsii (0.02 vs 0.03; z = 0.29, P = 0.77).
Table 1 Percentage (mean number of individuals) of echinoids occupied by decapods, fishes, and ectosymbionts at Los Cabos, Baja California Sur, Mexico.
| Associates | Echinoids | ||||||
| Eucidaris thouarsii < 4 m (n = 31) | Eucidaris thouarsii 5-20 m (n = 33) | Diadema mexicanum < 4 m (n = 370) | Diadema mexicanum & Centro-stephanus coronatus 5-20 m (n = 95) | Toxopneustes roseus < 4 m (n = 46) | Tripneustes depressus < 4 m (n = 116) | Echinometra vanbrunti < 4 m (n = 367) | |
| Decapods (combined) | 3.2 (0.03) | 3.0 (0.03) | 16.2 (0.21) | 2.1 (0.02) | - | - | 0.5 (0.005) |
| Tuleariocaris holthuisi | - | - | 0.3 (0.003) | - | - | - | - |
| Petrolisthes sanfelipensis | 3.2 (0.03) | 3.0 (0.03) | 9.5 (0.11) | 2.1 (0.02) | - | - | - |
| Calcinus californiensis | - | - | 3.2 (0.04) | - | - | - | - |
| Percnon gibbesi | - | - | 3.0 (0.04) | - | - | - | 0.3 (0.003) |
| Plagusia sp. | - | - | 0.3 (0.003) | - | - | - | 0.3 (0.003) |
| Fishes (combined) | 16.1 (0.19) | 24.2 (0.24) | 42.4 (0.86) | 24.2 (0.51) | 2.2 (0.02) | - | 2.2 (0.02) |
| Arcos erythrops | - | - | 0.3 (0.008)* | ** | - | - | - |
| Gobiesox adustus | - | - | 0.5 (0.005)* | ** | - | - | - |
| Tomicodon myersi | - | - | 0.8 (0.008)* | ** | - | - | - |
| Doryrhamphus excisus | - | - | - | 3.2 (0.05) | - | - | - |
| Apogon retrosella | - | 3.0 (0.03) | 1.1 (0.01) | 3.2 (0.05) | - | - | - |
| Microspathodon dorsalis | - | - | 0.5 (0.005) | - | - | - | - |
| Stegastes flavilatus | - | - | 0.3 (0.003) | - | - | - | - |
| Thalassoma lucasanum | 3.2 (0.03) | - | 7.3 (0.11) | 4.2 (0.06) | - | - | 0.3 (0.003) |
| Axoclinus storeyae | - | - | 1.6 (0.03)* | ** | - | - | 0.3 (0.003) |
| Cirriemblemaria lucasana | - | - | 0.5 (0.005) | - | - | - | - |
| Tigrigobius limbaughi | - | - | - | 4.2 (0.08) | - | - | - |
| Tigrigobius puncticulatus | 9.7 (0.13) | 15.2 (0.15) | 37.3 (0.56) | 7.4 (0.15) | 2.2 (0.02) | - | 1.6 (0.02) |
| Bathygobius ramosus | - | - | 0.3 (0.003) | - | - | - | - |
| Canthigaster punctatissima | 3.2 (0.03) | - | 1.1 (0.01) | 6.3 (0.06) | - | - | - |
| Unidentified fishes | - | 3.0 (0.03) | 8.4 (0.11) | 5.3 (0.05) | - | - | - |
| Ectosymbionts (decapods and fishes combined) | 19.4 (0.23) | 27.3 (0.27) | 51.9 (1.07) | 26.3 (0.53) | 2.2 (0.02) | - | 2.7 (0.02) |
* Additional individuals possibly observed but unidentified. ** Possibly observed but unidentified.
Five species of decapods associated with D. mexicanum; of these, five species associated with 16.2 % of D. mexicanum in shallow water and one species associated with 2.1 % of the diadematids (none confirmed with C. coronatus) in deeper water (Table 1). Decapods associated more frequently with D. mexicanum in shallow water than with the diadematids in deeper water (X2 = 13.0, d.f. = 1, P < 0.001), with significantly more decapods per echinoid in shallow water than in deeper water (0.21 vs 0.02; z = 3.62, P < 0.001; Table 1). The number of decapods per echinoid was positively correlated with spine length of D. mexicanum in shallow water (rs = 0.13, P = 0.01) but not with spine length of the diadematids in deeper water (rs = 0.19, P = 0.06), and was positively correlated with spine length of the diadematids when all data were combined (rs = 0.19, P < 0.001; Fig. 1).
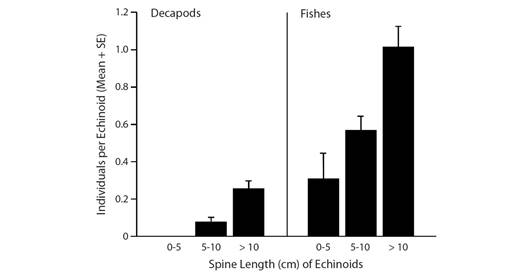
Fig. 1 Mean number of decapod and fish individuals associated with Diadema mexicanum and Centrostephanus coronatus hosts of different size categories (data combined for shallow and deeper water) at Los Cabos, Mexico.
One species of decapod associated with 3.2 % of E. thouarsii in shallow water and with 3.0 % in deeper water, with an average of 0.03 decapods per echinoid in both shallow and deeper water (Table 1). Two species of decapods associated with 0.5 % of E. vanbrunti, with a mean of 0.005 decapods per echinoid (Table 1).
We observed 76 individuals of five decapod species associated with echinoids in shallow water and two decapods of one species in deeper water. The porcellanid crab Petrolisthes sanfelipensis was the most common decapod associate of echinoids, comprising 56.6 % of the decapods observed in shallow water and all of the decapods observed in deeper water (Table 1). It associated with two species of echinoids, E. thouarsii and D. mexicanum, but did not differ between the two species in its frequency of association in shallow water (Fisher exact P = 0.34; insufficient data for deeper water; Table 1). It usually occurred alone (83.8 %), but sometimes as a duo (13.5 %) and rarely as a trio (2.7 %). All individuals were underneath the spines of echinoids (Fig. 2A); none were observed apart from echinoids.

Fig. 2 Decapod crustaceans associated with the echinoid Diadema mexicanum at Los Cabos, Mexico: A. Petrolisthes sanfelipensis; B. Calcinus californiensis; C. Percnon gibbesi; D. Plagusia immaculata or P. squamosa. Photographs by F. E. Hayes.
The diogenid hermit crab Calcinus californiensis accounted for 21.1 % of the decapods associated with echinoids in shallow water; it was not encountered in deeper water. It associated only with the longest-spined echinoid, D. mexicanum (Table 1), occurring either alone (66.7 %) or with a second individual (33.3 %). It was usually underneath or beside the spines of echinoids (Fig. 2B) and quickly retreated deeper under the spines when threatened. We occasionally saw individuals apart from echinoids.
The plagusiid crab Percnon gibbesi comprised 18.4 % of the decapods associated with echinoids in shallow water; it was not encountered in deeper water. It associated only with the longest-spined echinoid, D. mexicanum (Table 1), occurring either alone (81.8 %) or with a second individual (18.2 %). It was usually beside the spines of echinoids and quickly retreated under the spines when threatened (Fig. 2C). We occasionally saw individuals apart from echinoids.
We observed two unidentified individuals of the plagusiid crab Plagusia immaculata or Plagusia squamosa (Hendrickx, 1996; Schubart & Ng, 2000; Schubart et al., 2001), which accounted for 2.6 % of the decapods associated with echinoids in shallow water; none were encountered in deeper water. It associated with two species of echinoids (Table 1), D. mexicanum and E. vanbrunti, occurring alone on both occasions under the spines of the echinoid (Fig. 2D). We did not observe it apart from echinoids.
A small and dark palaemonid shrimp, presumably Tuleariocaris holthuisi (which had been previously collected within the study area; Wicksten & Hernández, 2000), was briefly observed on a spine of a D. mexicanum (Table 1) at Playa el Chileno, accounting for 1.3 % of the decapods observed associated with echinoids. It was not observed apart from echinoids.
Fish-echinoid associations: Fourteen species of fishes associated with echinoids (Table 1). The frequency of echinoids hosting fishes in shallow water (< 4 m) differed significantly among the five species of echinoids (X2 = 283.3, d.f. = 4, P < 0.001) and was greatest for the longest-spined species, D. mexicanum, which was 2.6 times higher than that of any other echinoid species (42.4 % vs 16.1 % for E. thouarsii; Table 1). The mean number of fishes per echinoid in shallow water was 4.5 times higher for the longest-spined species, D. mexicanum, than that of any other echinoid species (0.86 vs 0.19 for E. thouarsii; H = 381.6, P < 0.001; Table 1). The frequency of echinoids hosting fishes in deeper water (5-20 m) did not differ significantly between the diadematids and E. thousarsii (24.2 % vs 24.2 %; X2 = 0.0, d.f. = 4, P = 1.0; Table 1) and the mean number of fishes per echinoid in deeper water did not differ significantly between the diadematids and E. thouarsii (0.52 vs 0.24; z = 0.25, P = 0.80).
All fourteen species of fishes associated with D. mexicanum; of these, 12 species associated with 42.4 % of D. mexicanum in shallow water and six species associated with 24.2 % of the diadematids (none confirmed with C. coronatus) in deeper water, averaging 0.86 fishes per echinoid in shallow water and 0.51 fishes per echinoid in deeper water (Table 1). Fishes associated significantly more frequently with D. mexicanum in shallow water than with the diadematids in deeper water (X2 = 9.8, d.f. = 1, P = 0.001), with significantly more fishes per echinoid in shallow water than in deeper water (0.86 vs 0.51; z = 3.30, P = 0.001; Table 1). The number of fishes per echinoid was not correlated with spine length of D. mexicanum in shallow water (rs = 0.08, P = 0.12) or with spine length of the diadematids in deeper water (rs = -0.03, P = 0.76), but was positively correlated with spine length when all data were combined (rs = 0.13, P = 0.005).
Five species of fishes associated with E. thouarsii; of these, three species associated with 16.1 % of the echinoids in shallow water and three species associated with 24.2 % of the echinoids in deeper water (Table 1). The frequency of fishes associated with E. thouarsii did not differ between shallow and deeper water (X2 = 0.25, d.f. = 1, P = 0.62) and the mean number of fishes per echinoid did not differ between shallow and deeper water (0.2 vs 0.2; z = 0.71, P = 0.48; Table 1).
Three species of fishes associated with 2.2 % of E. vanbrunti, with an average of 0.02 fishes per echinoid (Table 1). Only one species of fish associated with 2.2 % of T. roseus, with an average of 0.02 fishes per echinoid (Table 1). No fish was observed associated with T. depressus.
We observed 335 individuals of 12 fish species associated with echinoids in shallow water and 55 fishes of six species in deeper water. Three species of gobiesocid clingfishes associated with echinoids: Arcos erythrops, Gobiesox adustus, and Tomicodon myersi. All three species associated exclusively with the longest-spined echinoid, D. mexicanum, together comprising 2.4 % of the fishes associated with echinoids in shallow water (Table 1). Additional individuals were possibly observed in both shallow and deeper water but were not identified to species. Only adults were observed. All were beside or underneath the spines of echinoids (Fig. 3A). None were observed apart from echinoids, but these cryptically colored species are easily overlooked.
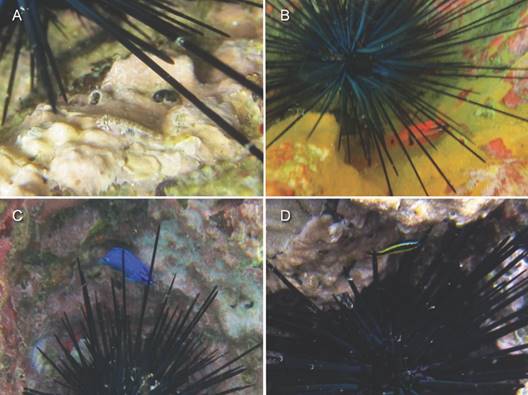
Fig. 3 Fishes associated with the echinoid Diadema mexicanum at Los Cabos, Mexico: A. Tomicodon myersi; B. Apogon retrosella; C. juvenile Microspathodon dorsalis; D. juvenile Thalassoma lucasanum. Photographs by F. E. Hayes.
The syngnathid pipefish Doryrhamphus excisus did not associate with echinoids in shallow water but it comprised 9.1 % of the fishes associated with echinoids in deep water, only with the longest-spined species of echinoid, D. mexicanum (Table 1). Only adults were observed, occurring alone (33.3 %) or in pairs (66.7 %). None were observed apart from echinoids.
The apogonid cardinalfish Apogon retrosella comprised 1.2 % of the fishes associated with echinoids in shallow water and 10.9 % in deeper water (Table 1). In both shallow and deeper water, it associated almost exclusively with the longest-spined species of echinoid, D. mexicanum, but it did not associate more frequently with the diadematids in either shallow or deeper water (Fisher exact P = 0.15; Table 1). One individual associated with an E. thouarsii in deep water (Table 1). It was usually alone (87.5 % for all echinoids combined) but one D. mexicanum hosted three individuals. Both adults and juveniles associated with echinoids, sheltering beside or under the spines (Fig. 3B). We often observed individuals in crevices apart from echinoids.
Two species of pomacentrid damselfishes, Microspathodon dorsalis and Stegastes flavilatus, associated exclusively with the longest-spined species of echinoid, D. mexicanum, accounting for 0.6 % and 0.3 %, respectively, of the fishes associated with echinoids in shallow water (Table 1). Most individuals, including all adults, did not associate with echinoids. Only a few juveniles < 6 cm long associated with echinoids, sheltering among the spines (M. dorsalis in Fig. 3C).
The labrid wrasse Thalassoma lucasanum was the second most common fish associated with echinoids, comprising 12.5 % of the fishes associated with echinoids in shallow water and 10.9 % in deeper water (Table 1). In shallow water it associated with three species of echinoids but it associated most frequently with the longest-spined species, D. mexicanum (X2 = 25.1, d.f. = 2, P < 0.001), at a rate 2.3 times higher than any other echinoid species (7.3 % vs 3.2 % for E. thouarsii; Table 1). It usually occurred alone (77.8 % for all echinoid species combined), with up to five individuals associated with D. mexicanum. In deeper water it associated only with D. mexicanum (and possibly C. coronatus), either alone (50 %) or in pairs (50 %). Its frequency of association with the diadematids did not differ between shallow water and deeper water (X2 = 0.71, d.f. = 1, P = 0.40). The number of individuals per echinoid was not correlated with spine length of the diadematids in either shallow water (rs = 0.10, P = 0.07) or deeper water (rs = 0.02, P = 0.82), but the correlation was significant for both depth categories combined (rs = 0.10, P = 0.04; Fig. 1). Most individuals, including all adults, did not associate with echinoids. We only observed juveniles < 6 cm long associated with echinoids, seeking shelter among the spines (Fig. 3D).
The tripterygiid triplefin Axoclinus storeyae comprised 3.3 % of the fishes associated with echinoids in shallow water. Additional individuals may have been observed but were not identified. Up to three associated only with the longest-spined species of echinoid, D. mexicanum (Table 1). All were adults and were sheltering beside or beneath the spines of echinoids. Some were observed apart from echinoids.
The chaenopsid blenny Cirriemblemaria lucasana accounted for 0.6 % of the fishes associated with echinoids in shallow water, exclusively with the longest spined-species of echinoid, D. mexicanum (Table 1). Both were solitary adults, sheltering beneath the spines of echinoids (Fig. 4A). None was observed apart from echinoids.
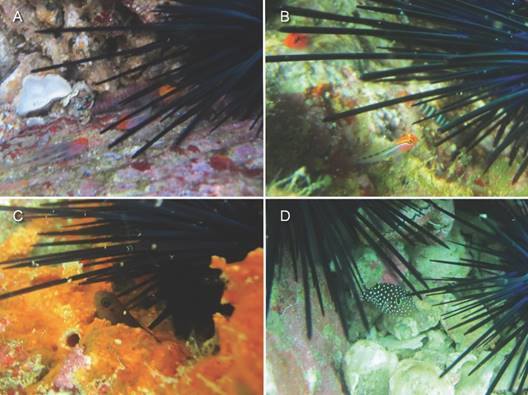
Fig. 4 Fishes associating with the echinoid Diadema mexicanum at Los Cabos, Mexico: A. Cirriemblemaria lucasana (above) and two Tigrigobius puncticulatus (below); B. two Tigrigobius puncticulatus (left) and Tigrigobius limbaughi (center); C. Bathygobius ramosus; D. juvenile Canthigaster punctatissima. Photographs by F. E. Hayes.
The gobiid goby Tigrigobius puncticulatus was the most common fish associated with echinoids, comprising 65.4 % of the fishes associated with echinoids in shallow water and 33.9 % of the fishes in deeper water (Table 1). In shallow water it associated with four species of echinoids, most frequently with the longest-spined species, D. mexicanum (X2 = 167.9, d.f. = 3, P < 0.001), at a rate 3.8 times higher than any other echinoid species (37.3 % vs 9.7 % for E. thouarsii; Table 1). It usually occurred alone (66.2 % for all echinoid species combined), with up to six individuals with D. mexicanum and up to two with E. thouarsii and E. vanbrunti. In deeper water it associated with two species of echinoids, E. thouarsii and D. mexicanum (none confirmed with C. coronatus), with similar frequencies (X2 = 0.95, d.f. = 1, P = 0.33), usually alone (83.3 % for both echinoid species combined) but with up to six individuals with D. mexicanum. It associated more frequently with the diadematids in shallow water than in deeper water (X2 = 30.2, d.f. = 1, P < 0.001), but it did not differ in its frequency of association with E. thouarsii between shallow water and deeper water (X2 = 0.08, d.f. = 1, P = 0.78; Table 1). The mean number of individuals per diadematid was significantly higher in shallow water than in deeper water (0.56 vs 0.15; z = 5.51, P < 0.001). The number of individuals per echinoid was not correlated with spine length of the diadematids in either shallow water (rs = 0.02, P = 0.64) or deeper water (rs = 0.03, P = 0.75), but was significantly correlated with spine length when all data were combined (rs = 0.14, P = 0.002). Individuals of all ages usually occurred around the perimeter of echinoids and most quickly sought refuge under the spines when disturbed (Fig. 4A, Fig. 4B). Although we often observed individuals apart from echinoids, most were within 10 cm of echinoids. The only individual associated with the short-spined echinoid T. roseus retreated repeatedly underneath it.
Two other gobiid gobies associated much less frequently with echinoids. Tigrigobius limbaughi did not associate with echinoids in shallow water but it comprised 14.3 % of the fishes associated with the diadematids, under the spines, in deeper water (Table 1, Fig. 4B). Up to three individuals, all adults, associated with an echinoid. A single juvenile Bathygobius ramosus rested on top of an unidentified sponge under the spines of a D. mexicanum (Fig. 4C), representing only 0.3 % of the fishes associated with echinoids in shallow water (Table 1).
The tetraodontid puffer Canthigaster punctatissima accounted for 1.5 % of the fishes associated with echinoids in shallow water and 12.3 % in deeper water (Table 1). In both shallow and deeper water it associated almost exclusively with the longest-spined species of echinoid, D. mexicanum (none confirmed with C. coronatus), but one individual in shallow water shuttled back and forth between a D. mexicanum and an E. thouarsii (Table 1). It associated more frequently with the diadematids in deeper water than in shallow water (Fisher exact P = 0.007; Table 1). Most individuals, including all adults, did not associate with echinoids. We only observed juveniles < 6 cm long, always alone, seeking shelter within the spines of echinoids (Fig. 4D).
Ectosymbiont-echinoid associations: Multiple individuals and species of decapods and fishes often associated together with a single D. mexicanum host in shallow water (< 4 m), with maximum counts of three decapod individuals, two decapod species, eight fish individuals, four fish species, and two decapod species together with two fish species. In deeper water (5-20 m) we never observed more than one decapod or a decapod and fish together with an echinoid host, but we observed up to nine fish individuals and four fish species with a host.
The frequency of echinoids hosting ectosymbionts (decapods and fishes combined) in shallow water (< 4 m) differed significantly among the five species of echinoids (X2 = 310.6, d.f. = 4, P < 0.001) and was greatest for the longest-spined species of echinoid, D. mexicanum, which was 2.7 times higher than for any other echinoid species (51.9 % vs 19.4 % for E. thouarsii; Table 1). The mean number of ectosymbionts per echinoid in shallow water was 4.7 times higher for the longest-spined species, D. mexicanum, than that of any other echinoid species (1.07 vs 0.23 for E. thouarsii; H = 426.9; P < 0.001; Table 1). The frequency of echinoids hosting ectosymbionts in deeper water (5-20 m) did not differ significantly between the diadematids and E. thousarii (26.3 % vs 27.3 %; X2 = 0.0, d.f. = 1, P = 1.0; Table 1). The mean number of ectosymbionts per echinoid in deeper water did not differ significantly between the diadematids and E. thouarsii (0.53 vs 0.27; z = 0.18, P = 0.86). The number of ectosymbionts per echinoid was correlated with spine length of D. mexicanum in shallow water (rs = 0.13, P = 0.01) but not with spine length of the diadematids in deeper water (rs = 0.03, P = 0.80), and was correlated with spine length of the diadematids when all data were combined (rs = 0.20, P < 0.001).
Discussion
Decapod-echinoid associations: We recorded seven new records of association between decapods and echinoids. No species of decapod had been reported associating with the echinoid E. thouarsii; thus, our study adds P. sanfelipensis as an associate of E. thouarsii. Only three species of decapods, Clastotoechus gorgonensis, Stenorhynchus debilis, and T. holthuisi, had been reported associating with the echinoid D. mexicanum (Marin & Anker, 2009; Salas-Moya et al., 2021; Schoppe & Werding, 1996; Wicksten & Hernández, 2000). Our study adds P. sanfelipensis, C. californiensis, P. gibbesi, and Plagusia sp. as associates of D. mexicanum. No species of decapod had been reported associating with the echinoids C. coronatus or T. roseus, and one decapod, Gnathophylloides mineri, had been reported associating with the echinoid T. depressus (Salas-Moya et al., 2021; Wicksten & Hernández, 2000). We did not confirm any decapods associating with these three echinoid species. At least ten taxa of decapods had been reported associating with the echinoid E. vanbrunti (Vallejo, 2007), including Palaemon sp., Gnathophyllidae sp., Alpheus sp., C. gorgonensis, Petrolisthes armatus, Pachycheles sp., Megalobrachium sp., Mithraculus denticulatus, Xanthidae sp., and Pachygrapsus transversus. Our study adds P. gibbesi and Plagusia sp. as associates of E. vanbrunti.
Only one of the five species of decapods observed during this study, T. holthuisi, appears to associate exclusively with echinoids (Marin & Anker, 2009). However, Percnon gibbesi usually associates with echinoids of four species, especially Diadema antillarum, in the Caribbean Sea (Hayes et al., 2016), but it has not been reported associating with any echinoids in the Pacific Ocean or where it has been introduced in the Mediterranean Sea (Félix-Hackradt et al., 2018). Our observations confirm that P. gibbesi also associates with echinoids in the eastern Pacific Ocean. Our observations also reveal that P. sanfelipensis and C. californiensis frequently associate with echinoids. Although four of the five species of decapods in our study appeared to associate facultatively with echinoids, some associations may have been incidental rather than intentional.
Decapods in shallow water (< 4 m) associated with the longest-spined species of echinoid, D. mexicanum, more frequently and with a higher number of individuals per echinoid than for any other echinoid species. These results are consistent with controlled experiments demonstrating that the palaemonid shrimp Tuleoariocaris neglecta and the inachid crab Stenorhynchus seticornis, when given a choice of several echinoid species, preferred to associate with the longest-spined species, D. antillarum (Castro, 1978; Joseph et al., 1998). Our results are also consistent with an observational study demonstrating that decapods in Honduras preferred to associate with the longest-spined species of echinoid, D. antillarum, more frequently and with a higher number of individuals per echinoid than that of any other echinoid species (Hayes et al., 2019). In deeper water (5-20 m), the frequency of association and the number of decapods per echinoid inexplicably were not significantly greater for the longest-spined diadematids. However, the number of decapods per echinoid was positively correlated with spine length of the diadematids when all data were combined. These results contrast with a previous study in which the decapods P. gibbesi and S. seticornis did not prefer to associate with the longest-spined individuals of D. antillarum (Hayes et al., 1998).
Fish-echinoid associations: Of the 14 fish species that we observed associated with echinoids, only two had been reported associating with echinoids: Tigrigobius puncticulatus associated with the echinoid E. thouarsii (Thomson et al., 2000), which we also documented, and G. adustus associated with the echinoid E. vanbrunti (Vallejo, 2007), which we did not document. An additional species of fish, Arcos decoris, has been reported associating with E. vanbrunti (Schoppe & Werding, 1996). We recorded 21 new records of association between fishes and echinoids, including: the fishes A. retrosella, T. lucasanum and A. hispidus associated with the echinoid E. thouarsii; the fishes A. erythrops, G. adustus, T. myersi, D. excisus, A. retrosella, M. dorsalis, S. flavilatus, T. lucasanum, A. storeyae, C. lucasana, T. limbaughi, T. puncticulatus, B. ramosus, and A. hispidus associated with the echinoid D. mexicanum; the fish T. puncticulatus associated with the echinoid T. roseus; and the fishes T. lucasanum, A. storeyae, and T. puncticulatus associated with the echinoid E. vanbrunti.
None of the 14 species of fishes are known to associate exclusively with echinoids, indicating that the associations are facultative rather than obligatory. All fishes associated with echinoids were small, < 6 cm in body length. Only small fishes are capable of retreating underneath or among the spines of echinoids, potentially benefitting from the protective spines of echinoids (Karplus, 2014). In five of the 14 fish species only juveniles associated with echinoids. Many larger fish species are known to associate facultatively with echinoids only as juveniles (e.g., Giglio et al., 2017; Hayes et al., 2019; Karplus, 2014).
Fishes in shallow water (< 4 m) associated with the longest-spined species of echinoid, D. mexicanum, more frequently and with a higher number of individuals per echinoid than for any other echinoid species. These results are consistent with an observational study demonstrating that fishes in Honduras preferred to associate with the longest-spined species of echinoid, D. antillarum, more frequently and with a higher number of individuals per echinoid than that of any other echinoid species (Hayes et al., 2019). In deeper water (5-20 m), the frequency of association and the number of fishes per echinoid inexplicably were not significantly greater for the longest-spined species, D. mexicanum (and C. coronatus). However, the number of fishes per echinoid was positively correlated with spine length of the diadematids when all data were combined.
Echinoids are preyed upon by a variety of fish species, with at least seven fish species known to prey on D. mexicanum (Alvarado et al., 2015). Small fishes seeking shelter under or among the spines of echinoids do not constitute an existential threat to the echinoids (Karplus, 2014), but some fishes prey upon the podia and pedicillaria of echinoid hosts (Briggs, 1955; Dix, 1969; Pfaff, 1942; Russell, 1983; Sakashita, 1992; Teytaud, 1971). Although we did not observe any predation on echinoids by fishes, predation on podia and pedicillaria may provide an alternative explanation for why fishes associate with echinoids.
Ectosymbiont-echinoid associations: Our data indicate that multiple individuals and species of decapods and fishes often associate together with a single echinoid host, all presumably seeking shelter to reduce the risk of predation. Although we did not observe any interspecific behavior interactions among decapods, fishes, and their echinoid hosts, we cannot exclude the possibility of competitive or predatory interactions. Such potential interspecific behavioral interactions merit further study.
Decapods, fishes, and both groups combined associated more frequently with echinoids in shallow water (< 4 m) than in deeper water (5-20 m). A previous study found no difference between the association of decapods with echinoids in shallow water and deeper water in Honduras (Hayes et al., 2016), but P. gibbesi associated more frequently with D. antillarum in shallow water and S. seticornis associated more frequently with D. antillarum in deeper water. Our results suggest that predation on decapods and fishes may be more intense in shallow water than in deeper water, but more investigation is needed before any conclusions are warranted.
Our results support the hypothesis that decapods and fishes associate most frequently with echinoid individuals and species with the longest spines, presumably to reduce the risk of predation. Other factors may also affect the frequency and abundance of ectosymbionts associated with echinoids, such as the availability of nutrients (which influences size of echinoids), type of substrate, shape of substrate (cracks and cavities may attract more ectosymbionts), and water currents.
Ethical statement: The authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 


