Introduction
The North Pacific coast of Costa Rica contains side-by-side two of the world’s most endangered forest types; tropical dry forests and mangrove estuaries (Cortés, 2014; Duke et al., 2007; Janzen, 1988; Miles et al., 2006). Outside of the protected areas of Santa Rosa National Park (SRNP) (10° 50’ 35.2” N - 85° 42’ 13.0” W) and the Horizontes Experimental Forestry Station (10° 42’ 56.2” N - 85° 34’ 0.7” W), tracts of tropical dry forest in this region are fragmented into small and isolated pockets, often converted to land for cattle haciendas or hotel development (Janzen, 1988; Jiménez, 2004). Mangrove estuaries are afforded protection against impacts and removal via Costa Rica’s National Wetland Policy, however these habitats and their surrounding areas remain at risk of degradation as human coastal populations grow (Jiménez, 2004; Slobodian & Badoz, 2019). In the North Pacific, as unprotected tropical dry forest is removed around mangrove estuaries, remaining forests of both types become increasingly isolated. Patches of intact forest may become ‘islands’ of biodiversity in which a surprisingly high number of species may still persist, but may be exposed to an increasing host of issues. This includes decreased gene flow, edge effects, and low population sizes, all of which can contribute to the eventual decline of species richness and ecosystem health (Andren, 1994; Saunders et al., 1991; Turner & Corlett, 1996).
Globally, survey efforts for mangrove forest fauna are amongst the poorest for any habitat, despite their potential to act as population refuges in areas of high anthropogenic disturbance and their importance to a variety of charistmatic megafauna globally (Barlow et al., 2011; Nagelkerken et al., 2008; Nowak, 2013; Rog et al., 2017; Thompson & Rog, 2019). Central America has one of the highest alpha-diversities of terrestrial vertebrates utilizing mangroves of any region globally (Rog et al., 2017), however few if any studies have examined the terrestrial vertebrate communities of Northern Pacific Costa Rican mangroves or tropical dry forests oustide of protected areas such as SNRP (Bonoff & Janzen, 1980; Escobar-Lasso et al., 2017; Montalvo et al., 2015; Montalvo et al., 2020).
Even fewer studies exist on the composition of vertebrate communities within the tropical dry forest and mangrove estuary habitat matrix, and how they may utilize both habitats over time, despite their established conservation value and unique characteristics (Jiménez, 2004; Luther & Greenberg, 2009; Nagelkerken et al., 2008; Rog et al., 2017; Zamora-Trejos & Cortés, 2009). Both mangrove estuaries and tropical dry forests are dynamic environments, changing seasonally (e.g. wet and dry seasons) and in the case of mangroves, daily (e.g. tidal fluctuations), and may contain unique assemblages of vertebrate fauna adapted to such challenging environments (Jiménez, 2004; Zamora-Trejos & Cortés, 2009).
To address this gap in our knowledge of the vertebrate community of the tropical dry-mangrove forest matrix, we deployed a non-fixed duration camera trapping grid within and between these habitats in a remote, unprotected area of North Pacific Costa Rica. We aimed to sample across habitat types during the late wet to early dry seasons in order to determine how species detections may change spatially and temporally in this environment. Our goal was to create a species inventory for this unprotected area which may be valuable for conservation management as well as improve our understanding of how vertebrate species may persist in this unique and threatened ecosystem.
Materials and methods
Study Site: The Cabuyal estuary (10° 40’ 21.6” N - 85° 39’ 06.9” W) in the Nacascolo District of Guanacaste, Costa Rica is an approximately 60 ha intertidal estuary system characterized by mangrove swamp surrounded by tropical dry forest (Cordero-Umaña & Santidrián-Tomillo, 2020; Córdoba-Muñoz et al., 1998; Yaney-Keller et al., 2019). The area receives approximately 1 400 mm of rainfall per year, virtually all during the rainy season (May-November), and is fed by a seasonal tributary of the Tempisque river (Córdoba-Muñoz et al., 1998). A much smaller (approximately 1.75 ha), intertidal estuary known as “Zapotillal” (10°39’29.3” N - 85°40’10.9” W) sits approximately one km to the South of Cabuyal, on the Northern Peninsula Papagayo. The surrounding area is a mixture of tropical dry forest and agricultural land with few roads and paths. While the mangrove swamps remain fairly undisturbed, areas of tropical dry forest have experienced clearing and development in the past.
Camera Stations: A total of 13 different automatic camera trap stations were placed in a non-random location matrix with no fixed time limits on trap duration within and around the estuaries and forests of Cabuyal and Zapotillal between September 2017 and February 2018 (Fig. 1). Camera traps photograph wild animals via the use of a passive infrared sensor which detects movement and differential heat signatures from a subject and its surrounding environment (Mohd-Azlan et al., 2016; Swann et al., 2004). Trap locations were chosen to maximize species detections based on presence of animal signs (e.g. tracks and scat), proximity to trails and water features and distance from other camera traps (minimum distance = 0.04 km, maximum distance = 1.5 km, average distance between traps = 0.5 km). Traps were checked approximately every ten to fourteen days. If a camera did not yield photo captures within the first sampling period, it was removed from its location, and placed in a more suitable location. Cameras were also moved if the camera was likely to be tampered with.
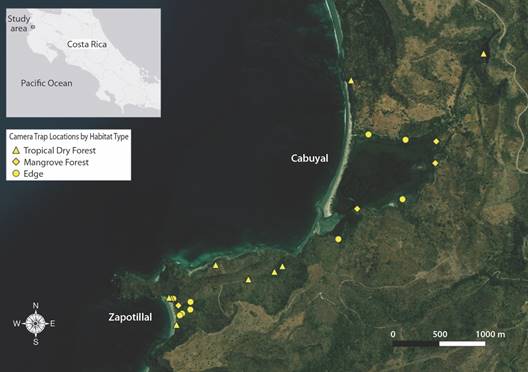
Fig. 1 Map of camera trap locations (n = 22) by habitat type in the Cabuyal and Zapotillal estuaries. Contour lines represent elevation. Map data from ESRI imagery.
All cameras were initially set to take a series of three photographs at the default trigger rate of the camera, if cameras began yielding large (> 1 000) sets of images in a single sampling period, they were switched to take a single photograph. If sampling yielded a species or behavior of interest, cameras were set to capture video. Cameras were mounted on trees, logs, or fence posts at approximately 30 cm height when possible and 1.5 to 3 m from the desired area to be sampled, following recommendation from TEAM Network (2011). Camera photos were downloaded and checked in the field to reduce the amount of time cameras remained out of operation.
Photograph Analysis: The number of trap days per camera per location, defined as 24-hour periods over which camera traps remained in operation, was calculated from the number of days cameras were in operation at each location, subtracting days of malfunction.
Camera trap locations were put into one of three habitat types in post-hoc analysis; edge, mangrove forest, and tropical dry forest. These categories were based on two metrics: 1) the abundance and species of mangrove vegetation around the trap location and 2) the presence of natural standing water visible within 10 m of the camera trap location. Mangrove species tend to form fairly monotypic stands in generally well-defined geographic zones (Ellison 2002; Snedaker, 1982). In dry, coastal zones similar to the Cabuyal and Zapotillal estuaries, Avicennia germinans is found in higher elevation sites that are drier and hypersaline, while species of the genus Rhizophora spp. tend to occur at lower elevation, near water channels and in less saline soil (Castañeda-Moya et al., 2006; Delgado et al., 2001; Samper-Villarreal et al., 2012). Based on Yaney-Keller et al., (2019), Laguncularia racemosa distribution in Cabuyal and Zapotillal estuaries forms an intermediate zonation remaining generally more abundant near fresh-water inputs, similar to other regional mangrove forests (Delgado et al., 2001; Samper-Villarreal et al., 2012). Mangrove forest locations were thus defined as those with a dominant vegetation type of R. racemosa and/or L. racemosa and located within 10 m of standing water that remained for the majority of the study duration. Edge locations were classified as those containing a mixture of either L. racemosa and/or A. germinans and/or tropical dry forest vegetation and did not meet the standing water criterion. Tropical dry forest contained only typical tropical dry forest vegetation and no mangroves. Mangroves were identified based on Tomlinson (1986). Camera trap days were totaled and averaged across camera locations per habitat type. The number of traps in each habitat type was proportional to its relative area in the region, accessibility, and amount of animal sign seen, and all habitat types were sampled simultaneously.
Two full analyses of the photos and videos taken were performed to identify positive faunal detection events (n = 2 648). For the purposes of this analysis, a three-photo sequence or a single photo were considered single events, as were individual videos regardless of length. Each photo and video taken was examined to determine whether it represented an animal detection or not. Positive detection events were defined by 1) a photo or video containing an individual or group of animals of a single species and 2) an individual of a species that does not re-occur within a single 60-min period, based on methodology following O’Brien et al., (2003), Yasuda (2004), and Meek et al., (2014). Species were identified and categorized into herbivore, carnivore, and omnivore foraging guilds using Stiles and Skutch (1989), Leenders (2001), and Reid (2009). Species richness and the number of unique species was calculated for all habitat types.
A rarefaction analysis was used to compare species detections and trapping effort in the entire study and the different habitat types sampled. In sample-based rarefaction analysis, rarefaction curves are created from the means of species accumulation curves, which are created by randomized and repeated re-sampling of detections from a species occurrence dataset (Gotelli & Colwell, 2001). This allows the expected number of species in a given sampled area to be extrapolated over increased sampling and time. As rarefaction curves reach an asymptote, they assess the adequacy of sampling effort in estimating complete diversity (Colwell et al. 2004; Colwell & Coddington, 1994; Gotelli & Colwell, 2001; Si et al., 2014). Sample-based rarefaction curves for each habitat type and mean species accumulation curve for total observed species were calculated in EstimateS and plotted against the total number of camera trap days (Colwell, 2005). One thousand runs were used for all randomizations, following Tobler et al., (2008).
Statistical analysis was used to determine the influence of month on species detections within each vertebrate class and habitat type. We used one-way repeated measure ANOVA’s with trap location as a random effect and Tukey HSD post hoc tests to compare camera trap detections between months within the edge, mangrove and tropical dry forest habitats. We tested for normality using Shapiro-Wilks and sphericity using Mauchly’s test of sphericity (Mauchly, 1940). All statistical tests were conducted with an a = 0.05 in R version 4.0.3 using base (R Core Team, 2020) and ‘rstatix’ (Kassambra, 2020) packages.
Results
A total of twenty-two camera trap locations were set in the Cabuyal and Zapotillal areas between September 2017 and March 2018. There was a mean number of 55 trap days per trap location, with a total of 1 498 trap days for the duration of the project. The lowest number of trap days in one location was seven days, while the one with the greatest number of trap days was 153 days (Table 1).
Table 1 Total number of camera trap locations, mean number of trap days per location and total number of trap days per habitat type in the Cabuyal and Zapotillal regions from September 2017 to March 2018.
| Habitat Type | Total Trap Locations | Mean Trap Days per Location | Total Trap Days |
| Mangrove Forest | 4 | 38.9 | 272 |
| Tropical Dry Forest | 8 | 62.7 | 557 |
| Edge | 10 | 59.9 | 669 |
| Total | 22 | 55.5 | 1 498 |
Throughout the total survey, 27 orders, 42 families and 70 species of vertebrate fauna were detected, not including domestic species (n = 4). Tropical dry forest, followed closely by edge, had the greatest total richness (Fig. 2). Avian richness was greatest in tropical dry forest, followed closely by mangroves, while edge habitat led in mammalian richness. Herpetofauna richness was equal, but very low, amongst all three habitats (Fig. 2). In total 36 species were found to uniquely occur in one of the sampled habitat types, over half of all species detected (Table 2). Tropical dry forest had the highest number of total avifauna and herpetofauna species not found in either of the other habitat types, while edge habitat had the greatest number of unique mammalian species (Table 2).
Table 2 Number of unique species not detected in other habitat types within vertebrate groupings (avian, mammalian and herpetofaunal) detected by camera traps in the Cabuyal and Zapotillal regions between September 2017 and March 2018.
| Habitat Type | Avian Species | Mammalian Species | Herpetofaunal Species | Total Unique Species |
| Edge | 4 | 6 | 0 | 10 |
| Mangrove Forest | 11 | 0 | 0 | 11 |
| Tropical Dry Forest | 13 | 2 | 1 | 16 |
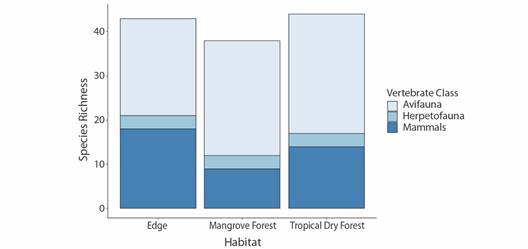
Fig. 2 Avian (n = 47), mammalian (n = 19), and herpetofaunal (n = 4) species richness of the Cabuyal & Zapotillal regions by habitat type (edge, mangrove forest and tropical dry forest).
Between all vertebrate species, carnivores made up the greatest percentage of species detected in the edge (49 %) and mangrove forest (56 %), while herbivores were the lowest in all forest types (23 %) (Fig. 3). Amongst avifauna, carnivores made up the majority of edge (68 %) and mangrove (69 %) and a large percentage of tropical dry forest (41 %) richness (Fig. 3). Omnivores made up the majority of mammalian species detected in all habitat types (Fig. 3). Among herpetofauna species, carnivore, omnivore, and herbivores were equivalently detected in edge and mangrove forest, while only carnivores (67 %) and herbivores (33 %) were detected in tropical dry forest (Fig. 3).
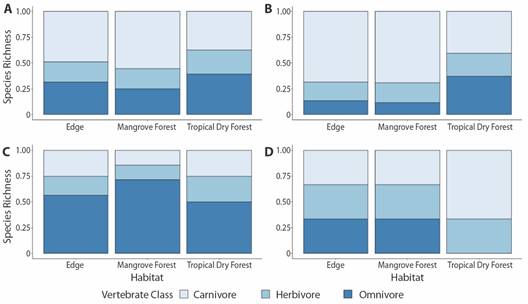
Fig. 3 Percentage of (A) total (n = 70), (B) avian (n = 47), (C) mammalian (n = 19) and (D) herpetofaunal species (n = 4) detected in carnivore, herbivore and omnivore foraging guilds within edge, mangrove forest and tropical dry forest habitat types of the Cabuyal and Zapotillal regions.
Rarefaction analysis showed that differences in sampling effort likely accounted for some of the differences in richness between the habitat types (Fig. 4). Tropical dry forest habitat rarefaction curves and 95 % confidence intervals (CI) indicate that the sampling effort of 669 trap days was likely sufficient to account for the majority of species within this habitat type (Fig. 4). Edge habitat sampling was somewhat less sufficient than tropical dry forest sampling, although the 95 % CI of relative species richness overlapped between these habitats and accounted for 90 percent of the species in the area (Fig. 4). Mangrove habitat was the least sampled type both in duration and number of locations, and rarefaction analysis revealed that sampling was likely inadequate to determine species richness in this habitat type (Fig. 4).
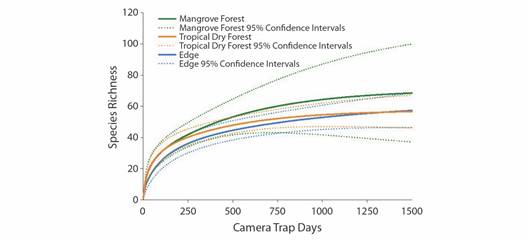
Fig. 4 Sample-based rarefaction curves for edge, mangrove forest, and tropical dry forest habitats extrapolated to 1 500 samples. Solid lines represent the rarefaction richness estimate, while dashed lines indicate 95 % confidence intervals.
Shapiro-Wilks tests revealed that no mean detections per camera trap for any species grouping (all, avian, mammal or herpetofauna) met assumptions of normality. Means were then square root, log, or log (x + 0.5) transformed depending on whether they contained zero or non-zero count data (Berry, 1987), after which normality was met for all data sets (W > 0.90, P > 0.05). Data was automatically tested for assumptions of sphericity via Mauchly’s test with the anova_test function within the R package “rstatix”, and Greenhouse-Geisser corrections were automatically applied to F-values via the get_anova_table function when sphericity was not met (Bathke et al., 2009; Kassambra, 2020). Mixed-effects one-way repeated measure ANOVA’s revealed statistically significant differences in mean detections per camera trap across months for all species within the edge (F (5.23) = 2.76, p = 0.043) and tropical dry forest (F (5.21) = 3.34, p = 0.022), but not mangrove forest (F (5.8) = 3.63, p = 0.052) (Fig. 5A). For all species, Tukey HSD post-hoc comparisons indicated significant increases in detections within the tropical dry forest between October and January (p = 0.045) and October and February (p = 0.024), but not between September, November and December. (Fig. 5A). No significant differences in avian species detections between months could be ascertained in any habitat type (Fig. 5B). Statistically significant differences were found in mammalian species detections between months within the edge (F (5.19) = 5.00, p = 0.004) and tropical dry forest (F (5.17) = 4.17, p = 0.012), but not mangrove forest (F (5.5) = 2.47, p = 0.172) (Fig. 5C). Tukey HSD post-hoc comparisons indicated significant increases in edge mammalian detections between September and December (p = 0.031), September and January (p = 0.02), and October and January (p = 0.031). Mammalian detections significantly increased in tropical dry forests between October and January (p = 0.047) and October and February (p = 0.008), but not between September, November and December. (Fig. 5C). Statistically significant differences in herpetofauna detections were found between months within the edge habitat (F (5.13) = 3.77, p = 0.025), but not within tropical dry (F (5.7) = 2.69, p = 0.115) or mangrove forests (F (5.3) = 0.92, p = 0.564).
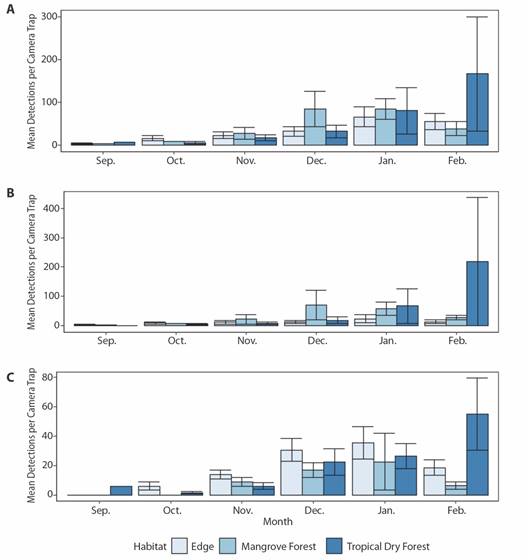
Fig. 5 Average A. total, B. avian, and C. mammal species detections per camera trap within edge, mangrove forest, and tropical dry forest habitats between September 2017 and February 2018 in the Cabuyal and Zapotillal regions. Error bars represent standard error from the mean.
Over 1 498 trap days, a total of 2 648 positive detection events were registered. The species with the highest number of positive detection events for the entire camera trapping season and positive detection events per day was Nyctanassa violacea (yellow-crowned night heron) (Appendix 1). The species with the largest group size photographed together in a single positive detection event (the minimum number of individuals potentially photographed in the detection area) was Nasua narica (white-nosed coati) (Appendix 1). Ten species protected under the Costa Rican environmental law, Decreto Número 32 633-MINAE, Artículos 26 y 29 (2005), which protects species with decreasing populations (article 26) and those that are endangered (article 29), were detected in the study, as well as four classified as “vulnerable” or “near threatened” under the International Union for the Conservation of Nature’s Red List (see Appendix 2 and Appendix 3 for lists of species).
Discussion
Tropical dry forests and associated mangrove estuaries are considered more species poor than other forest types in the tropics, so when combined with the historical deforestation and fragmentation in the area, the overall diversity of species found in this region was surprising (Jiménez, 2004). The unique assemblages of species which occur within each habitat type as well as the high overall biodiversity between them highlights the importance of the tropical dry forest-mangrove forest matrix towards maintenance of biodiversity in the area.
While true absence of species from this site cannot be fully determined, the total sampling effort was relatively high for a camera trapping study of a site this size (Kelly, 2008; Kelly & Holub, 2008; Tobler et al., 2008, Trolle & Kéry, 2003). While edge habitat was sampled at more locations and over a longer overall period of time, rarefaction analysis points to a lower species richness in the edge than tropical dry forest, though increased sampling effort would likely yield increased detections (Fig. 4). At the maximum number of camera trap days for mangrove habitats, rarefaction curves indicated trapping effort was likely not sufficient in this habitat type to register all species present (Fig. 4). Increased sampling effort to at least 1 000 camera trap days per habitat type would likely capture a large majority of the species present in all habitat types in this region (Ahumada et al., 2011; Carbone et al., 2001; Tobler et al., 2008). Birds are the most diverse vertebrate species class, which may also explain the relatively high richness we found within and between habitat types in this group, though increased sampling may yield more detections (Fig. 1) (Rahbek & Graves, 2001). Further, increasing the number of trap locations and/or sampling techniques would likely also increase species detections, especially for species groupings not easily detected by camera traps, such as arboreal species and small mammals, birds and herpetofauna (Rog et al., 2020). To this point, two species listed as “vulnerable” by the IUCN (Allouta palliata, mantled howler monkey and Eupsittula canicularis, orange-fronted parakeet) and one listed as “endangered” (Amazona auropalliata, yellow-naped Amazon) were frequently encountered in the Cabuyal and Zapotillal region during our study in all three habitats, but were not detected by camera traps, illustrating the need for a diversity of sampling types to determine species presence/absence in this area (IUCN, 2020).
Seasonal Changes in Mean Detections: The increase in mean detections from the end of the wet to the beginning of the dry season in tropical dry and edge habitats we observed may be explained by increased movements of wildlife in the area or individuals entering it from other regions (Fig. 5). In tropical dry forests, seasonal shifts in behavior have been observed in several vertebrate groups (Asensio et al., 2012 Fuller et al., 2020; García et al., 2010; Herrera et al., 2018; MacKinnon, 2006; Maffei et al., 2005; Mosdossy et al., 2015; Nuñez-Perez & Miller, 2019; Valenzuela & MacDonald, 2002). These shifts may be especially pronounced in areas with high temporal and spatial scarcity in water and food resources, such as the tropical dry forests of Northwestern Costa Rica (Montalvo et al., 2015). These patterns are thought to be tied to a variety of biological factors, including foraging guild, energetic needs and capacity for group formation, and can vary greatly between species (Johnson et al., 2002; Reiss, 1988). However, underlying ecological mechanisms may also govern seasonal changes in vertebrate movements and density within and between these environments. Each year, food and water availability decreases dramatically in tropical dry forests with the onset of the dry season, as water availability is reduced to small pools of collected water and flowering plants bloom and shed their leaves to time fruit production with the later onset of rains (Stan & Sanchez-Azofeifa, 2019). This in turn will have bottom-up influences on individuals, populations and communities of vertebrate species in this environment (Boyle et al., 2020; Castro et al., 2018). Tropical storm Nate, which arrived in the Cabuyal-Zapotillal region in early October 2017, brought over 400 mm of rain in 48 hours (approximately 20% of 2017’s annual rainfall), and flooded much of the study area. Although camera trapping continued during this time and no equipment was damaged, it is likely that the event influenced animal movement patterns during and after. Long-term studies of species responses to both acute and persistent environmental changes in this region will be increasingly important as climate change is predicted to raise temperatures and alter the frequency and intensity of tropical storms and El Niño Southern Oscillation in Northwestern Costa Rica (Nakicenovic et al., 2000; Santidrián-Tomillo et al., 2012).
While no significant differences were found in the detection rates of species between months in mangroves in our study, mean detections per trap did increase from September through December and January for all, avian and mammalian species before decreasing in February (Fig. 5). Minimum and average monthly tide height followed this same pattern, peaking in January and declining in February (Appendix 4). Increasing tide heights during this period may change the availability of prey items, such as arthropods, fish and crustaceans, which may become prevalent throughout these months as higher tides bring these resources into areas of the mangrove forests more accessible to terrestrial species.
The Tropical Dry Forest - Mangrove Forest Matrix: Over half of all species detected in our study were found to occur within only one habitat type and all habitat types were found to host unique assemblages of species (Table 2). On the Pacific coast of Central America, mangrove forests may play a special role in harboring unique species, due to the contrast of the wet mangrove forest with the neighboring arid tropical dry forest (Luther & Greenberg, 2009; Woodcock & Woodcock, 2007). Woodcock y Woodcock (2007) found a greater diversity of bird species in North Pacific Costa Rican mangroves than neighboring tropical dry forests and similar to this study, different assemblages of species as well. This is thought to be due to different prey communities found between the mangrove and other habitat types (Lefebvre & Poulin, 1997; Luther & Greenberg, 2009; Woodcock & Woodcock, 2007). Our results appear to support this, as tropical dry forests supported a greater number of herbivorous and omnivorous bird species than neighboring edge and mangrove forests, which were dominated by carnivorous species (Fig. 3). While mangroves support a variety of prey for piscivores and other wetland birds, tropical dry forests host a greater variety of flowering plants, providing fruit, nectar, and insects for forest birds. On a regional scale, differences in species communities can be seen even between the same habitat types due to differences in physical factors between sites (e.g., rainfall, phenology, salinity) (Lefebvre & Poulin, 1997). Rog et al., (2020) found similar findings of unique terrestrial vertebrate assemblages within Australian mangrove forests. This emphasizes the need for increased research on faunal communities of all mangrove estuaries, regardless of size or abiotic characteristics, as well as the habitats around them to maintain biodiversity in the region.
The high amount of mammalian richness found in the edge habitat in our study could be due to the preferential use of human-created paths within these habitats or the transient use of mangroves as foraging grounds, but not as permanent residence (Luther & Greenberg, 2009). In the Cabuyal-Zapotillal region, mangroves are especially important considering the limited availability of neighboring intact forest, as mangroves may be refuges of habitat and resources, especially during the seasonal declines in neighboring dry forest productivity (Jiménez, 2004). Global reviews on facultative mammalian usage of wetlands suggest that very few mammalian species are restricted to mangroves for their entire life history (Hogarth, 2015; Luther & Greenberg, 2009; Rog et al., 2017). To truly asses mammal communities in mangroves, other survey techniques such as nocturnal transects and live or hair traps may be more optimal than camera traps (Rog et al., 2020). However, camera traps appear to have some utility across longer sampling periods in locations where they can be protected from inundation and where exposed banks or upland areas used for travel or foraging may be present.
Our results conform to the general global pattern reported by Rog et al., (2017) of high proportions of carnivorous and omnivorous terrestrial vertebrate usage of mangrove forests (Fig. 3). Herbivores may not find these environments ideal compared to neighboring tropical dry forests due to the high salt content of mangrove leaves, though they may opportunistically utilize these environments for nesting, roosting, or refuge while foraging in adjacent habitats (Kathiresan & Bingham, 2001; Luther & Greenberg, 2009). The high proportion of mammalian omnivores found between all habitat types is likely reflective of the adaptive nature of this foraging guild towards survival in this climatically and ecologically diverse region (Reid, 2009). It is important to note that the Cabuyal-Zapotilall region has also experienced significant deforestation and remaining forests are surrounded by and interspersed with small human habitations and pasture lands. These environments are known to influence community compositions, favoring generalist over specialist species, a pattern reflected in the relative abundance of species detected in our study (Appendix 1) (Prist et al., 2012). Release of meso-predator populations via decreases of larger predator (ie. jaguars and pumas) populations in the region also explains the high proportion of small carnivores and omnivores seen in this area, though large predator populations are rebounding in nearby SRNP (Crooks & Soulé, 1999; Montalvo et al., 2015).
Conservation Implications: Our findings highlight the need for further research into the vertebrate community of this region. While rich in biodiversity, the region is largely unprotected and faces imminent threat from development (Appendix 5). Though current legal frameworks protect mangroves and bordering habitats within a 150-meter buffer, surrounding tropical dry forests that support a high level of both unique and co-occurring biodiversity are left outside of these current protections (Slobodian & Badoz, 2019). Wide ranging species which may utilize mangrove and edge habitats during the wet and transitionary months most certainly leave the protective boundaries of these habitats in search of resources during the dry season. The single jaguar detected in this study was found to match a previously identified approximately three-year old female, last seen in SRNP two years prior and known to range within the park, indicating that wildlife dispersal between the park and this region does take place (Luis G. Fonseca, pers. comm.) (Appendix 6). Further studies integrating techniques from spatial ecology on vertebrates in the region will be useful for exploring the importance of the tropical dry forest-mangrove forest matrix for species in the region.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 


