Introduction
Neotropical seasonally dry forest -NSDF- species occur from Mexico to Argentina and in the Antilles (Banda et al., 2016). They have a disjunct distribution that is mainly associated with climatic conditions such as high temperatures (above 25 ºC), low rainfall regimes (< 1 800 mm), and strong rainfall seasonality (i.e., three to six months with less than 100 mm of precipitation (Banda et al., 2016). Pennington et al. (2009) suggest that these climatic conditions have shaped species ranges without immigrational subsidy and, therefore, they influence richness and patterns of endemism. Furthermore, according to the same authors climatic constraints have produced three features (emergent from phylogenetic studies) in taxa confined to this type of forest: (a) endemic species are confined to a single geographic areas (NSDF nuclei, sensuBanda et al. (2016)), which are (b) monophyletic and relatively old (predating the Pleistocene) and (c) sister species often tend to inhabit the same NSDF nuclei, suggesting phylogenetic niche conservatism (-PNC). Phylogenetic niche conservatism refers to lineages that tend to retain ancestral ecological characteristics over time (Angulo et al., 2012; Donoghue, 2008; Pennington et al., 2009).
Pennington et al. (2004) also suggested that taxonomic groups strongly associated to NSDF are important for understanding the evolutionary and biogeographic history of this biome. As suggested by floristic studies and based on their abundance, dominance, endemism, and interactions with other species, Fabaceae, Malvaceae, Cactaceae, Zygophyllaceae, and Capparaceae have been suggested to be important families in the NSDF (Banda et al., 2016; Herazo-Vitola et al., 2017). In particular, Neotropical Capparaceae include around 21 genera and 106 species that toward arid zones and tropical dry forest historically has been related (Cornejo & Iltis, 2012; Kers, 2003; Mercado-Gómez & Escalante, 2018) order to test the phylogenetic patterns of NSDF proposed by (Pennington et al., 2009) found 24-endemic species of Capparaceae distributed in five areas of endemism located in the NSDF nuclei (Central America and northern South America, Northern inter-Andean valleys, Central Andean coast, Central inter-Andean valleys, Apurimac-Mantaro, Piedmont, Misiones Central Brazil, and Caatinga). Furthermore, the endemic species of Capparaceae have been confined to NSDF nuclei and dated before to the Pleistocene, with sister species occurring in the same nucleus (Mercado-Gómez & Escalante, 2018). These findings support two of the three features proposed by Pennington et al. (2009), however, whether the evolutionary history of this family was shaped by PCN has not been tested.
The limited geographic distribution of Capparaceae and high endemic species in the NSDF nuclei also suggests that species of this family possibly originated and evolved mainly in these ecosystems (Mercado-Gómez & Escalante 2018; Mercado-Gómez & Escalante, 2020). However, Mercado-Gómez and Escalante (2018) also found an area of endemism located in the humid forest of the Panama Isthmus. In fact, Mercado-Gómez and Escalante (2020) through track analysis, identify biogeographic patterns related to both humid and dry forest and, therefore, the origins and relationship of Capparaceae with the NSDF is uncertain. In order to improve the knowledge of the geographic and climatic relationships of Capparaceae toward NSDF nuclei (Mercado-Gómez et al., 2020), carried out a climatic affinity analysis. These authors found three main climatic associations related toward three different forests dry, moist and wet. They also identified species with broad niches that occur in both dry, moist and wet forest (Capparidastrum frondosum (Jacq.) Cornejo & Iltis and Crateva tapia L.). These outcomes suggest that at first, species of similar ecological preferences have evolved largely independently towards moist, wet, and dry forests throughout the evolutionary history of this family and then phylogenetic climatic niche divergence (PCND) could have occurred. Whether the ancestors of Capparaceae emerged from areas climatically similar to those of NSDF remains unknown.
In order to understand how lineages of this family have evolved through space and time, a climatic niche evolution analysis was carried out to estimate the plausible ancestral climate of the area of origin of this family and to illuminate whether PNC or PCND explain the exceptional NSDF endemism patterns observed in neotropical Capparaceae (Mercado-Gómez & Escalante, 2018). Here we used a set of geographic (Broennimann et al., 2012; Warren et al., 2008) and phylogenetic analyses (Cooper et al., 2010; Münkemüller et al., 2015) for 24 species, in order to explain how the niche continuum process (PNC or PCND) explains the evolutionary history of this family. We evaluated the macroevolutionary climatic niche dynamics of Capparaceae to establish whether PNC (i.e., niche stasis) or PCND have been recurrent in the evolution of this arid climate adapted family. We used a combination of approaches including model-based phylogenetic comparative methods, numerical ecology, and ecological niche model in order to evaluate the climatic niche suitability of species through temporal and spatial scales.
Materials and methods
Species selection, distribution, and environmental data: A total of 24 neotropical species of Capparaceae were included in the analyses. We assembled a comprehensive data set of 4 700 distributional records compiled from several herbaria (e.g. CAUP, COL, CUVC, FMB, HUA, ICESI, JAUM, MEDEL, TOLI, UIS, UTMC) and online databases (e.g. NYBG, MO, MEXU and herbaria attached to the Species Link from SPF). All the acronyms of herbaria cited follow Holmgren et al. (1990), and an updated list is based on Thiers (2018). Furthermore, we checked different floras and publications (Cornejo & Iltis, 2008a; Cornejo & Iltis, 2008b; Cornejo & Iltis, 2008c; Cornejo & Iltis, 2008d; Cornejo & Iltis, 2009; Cornejo & Iltis, 2010a; Cornejo & Iltis, 2010b; Cornejo & Iltis, 2012; Cornejo et al., 2016; Lorea-Hernández, 2004; Mercado-Gómez et al., 2019; Mercado-Gómez & Morales-Puentes, 2020; Newman, 2007; Ruiz-Zapata, 2005; Ruiz-Zapata, 2006). We reviewed all data for accuracy and validity of the geographic coordinates through a Geographic Information System (GIS; QGIS 3.4). We eliminated all dubious localities. For all species, we removed records repeated in multiple sources, thus retaining only unique localities (at 1 km2 pixel). We displayed the geographic coordinates in decimal degrees, based on the WGS84 datum.
In order to obtain environmental data, we extracted climatic values from 19 variables of WorldClim (Hijmans et al., 2005) at a spatial resolution of 30” arc/degrees (approximately equal to 1 km2). We overlaid the climatic rasters on the occurrences of each species in order to extract the climatic values for each grid cell, resulting in a climatic data matrix for all species. We performed a principal component analysis (-PCA; Appendix 1A) and a non-metric multidimensional scaling (-NMDS, Appendix 1B) using the Pearson similarity index to analyze collinearity among bioclimatic variables (Dormann et al., 2013), and we selected those that best represent the realized climatic niche of Capparaceae (Appendix 1). We selected a final set of environmental variables that were revealed by the PCA and NMDS, including: temperature seasonality (BIO4), mean temperatures of the wettest (BIO8) and the driest (BIO9) quarters, annual precipitation (BIO12), and precipitations of the wettest (BIO16), the driest (BIO17), the warmest (BIO18) and coldest (BIO19) quarters. Part of these variables have been defined as the most significant NSDF and/or were used in previous studies (Mercado-Gómez et al., 2020; Prieto-Torres & Rojas-Soto, 2016).
Phylogeny: In order to infer a phylogenetic tree of Capparaceae we used 36 accessions of the plastid markers ndhF, matK, rbcL, and the mitochondrial one rsp3. From 36 accessions, we obtained 24 ingroups and 12 outgroup species to estimate the divergence times between lineages through fossil information and a previous dated phylogeny (Appendix 2). We aligned sequences using Atamisquea emarginata Miers ex Hook. & Arn., employing MUSCLE (Edgar, 2004) in Mesquite v3.1 (Diaz-Pulido & Díaz-Ruíz, 2003).
To estimate a Bayesian phylogeny, we determined the best substitution model for each dataset (loci) in JModelTest 2 (Darriba et al., 2012). We selected the best model using Akaike information criterion (AICc - Akaike, 1974). We used BEAST v2.3.0 (Bouckaert et al., 2014) to generate the phylogenetic estimation for all species. One cold and three incrementally heated MCMC chains were run for 10 000 000 generations. Settings included 2 runs and trees were sampled every 1 000 generations to minimize autocorrelation among samples. Post analyses were carried out in Tracer v1.7 (Rambaut et al., 2018) interpreting likelihood values against a number of generations, an ESS > 200 for the MCM chains and to check if the burn-in was adequately determined. Support was estimated by posterior probabilities. In addition, TreeAnnotator v1.8.4 was used to obtain the consensus trees, excluding the first 3 000 trees. Tracer v1.7 was used to check if chains from samples of two runs (which yielded similar results) combined and converged. The average standard deviation of split frequencies between the two runs was 0.01 for all data sets. We considered nodes with PP ≥ 0.90 to be well-supported.
We estimated the divergence times between lineages using the same parameters mentioned above for BI. We inferred a time-calibrated phylogenetic tree through an uncorrelated relaxed molecular clock with log-normal rate distribution and a Yule speciation model to estimate the times of divergence and their credibility intervals in BEAST (Bouckaert et al., 2014). Because Capparaceae has few fossil records and has no clear taxonomic affinity to current taxa, three different sources were used to date the calibrated phylogenetic tree: (i) We used primary calibration using Capparis multinervis Engelhardt, dated from the Pliocene (Berry, 1917), which has affinities to Quadrella angustifolia (Kunth) Iltis & Cornejo; (ii) We employed secondary dating calibration data from the Cardinal-McTeague et al. (2016) and Beilstein et al. (2010) phylogenetic analyses, which dated Capparaceae from 63 to 53 Mya. Furthermore (iii) we used four fossils from sister taxa of Brassicales for node calibrations in our molecular dating analyses, including: fossil of Dressiantha bicarpellata was assigned to Moringa oleifera Lam. (98.78 Mya) node (Gandolfo et al., 1998), Akania sp., was assigned to Akania bidwillii (Hogg) Mabb. (61 Mya) node (Lewis & Mccourt, 2004) and Palaeocleome lakensis (52.58 Mya) fossil was assigned to the node Cleome sp. (Cardinal-McTeague et al., 2016).
Niche quantification and comparison: Given that species distribution models must consider historical factors affecting the geographical distributions, we created an area for each species (or M sensu BAM diagram; see, (Soberon & Peterson, 2005) based on occurrence points and the intersection with terrestrial ecoregions (Olson et al., 2001) and biogeographical provinces (Morrone, 2014). We used these areas (M) to clip the climatic variables chosen for each species and to perform the niche similitude test using the environmental principal component analysis (PCA-env) proposed by Broennimann et al. (2012) in the Ecospat R package (Di Cola et al., 2016). We used the two first axes of the PCA-env calibrated on the entire environmental space of the species distribution area “M” to obtain the species climatic space through a grid of 100 x 100 cells in which each cell corresponded to a unique vector for the available environmental conditions in the study area (Broennimann et al., 2012). We utilized this information to estimate the similarity between the niches occupied by the species studied through Schoener’s D (Freshwater et al., 1994), implemented in the ENMTools R package (Warren et al., 2008).
Niche evolution analyses: To explore the relationships between climatic niche divergence and phylogenetic distances (maximum clade credibility tree) we used a Mantel test based on dissimilarity matrices. We employed the matrix of niche similitude from Schoener’s D metric outcomes and patristic distances calculated from PATRISTIC (Fourment & Gibbs, 2006) from our phylogenetic estimation. The Mantel test was run in the ade4 Package R (Bougeard & Dray, 2018).
We calculated the mean value of those selected climatic variables for each species and estimated the phylogenetic scaling parameters λ, κ and δ (Pagel, 2002) using the time-calibrated phylogenetic tree. All these parameters range from 0 to 1. When l takes values of 0 it indicates phylogenetic signal absence, while a λ near 1 shows a phylogenetic signal according to Brownian motion (Hernández et al., 2013). When κ takes values of 1, trait evolution is directly proportional to branch length; therefore, slow evolution is better supported (Hernández et al., 2013), whereas a κ near 0 indicates that character change is independent of evolutionary rate with rapid change during speciation or immediately following it suggested as is expected from punctuated equilibrium. Likewise, δ near 1 indicates gradual evolution. When δ is near 0, it indicates earlier trait evolution or ‘early burst’, it is indicative of adaptive radiation, while δ near 1 indicates later trait evolution, suggesting species specific adaptation (Hernández et al., 2013; Pagel, 2002). We investigated the mode of evolution along with each climatic niche component, fitting five alternative models of evolution: (1) A Brownian Motion (BM), (2) Ornstein-Uhlenbeck (OU), (3) Early Burst (EB), (4) White Noise (WN), and (5) Drift Models (DF). We chose the best-fit model using the AIC corrected for a small sample size (AICc). We carried out all evolutionary analyses in the Geiger package on R (Harmon et al., 2008).
We also mapped the climatic variables with a phylogenetic signal on the phylogeny through ancestral state reconstructions under maximum likelihood estimation, using Phytools 0.3-93 package on R (Revell, 2012). Furthermore, we generated traitgrams climatic variables with phylogenetic signal to explore the evolutionary trajectories of climatic niche through time. In order to assess if PCND could help explain the evolution of neotropical Capparaceae, we evaluated whether niche convergence was a recurrent pattern throughout Capparaceae evolution in dry and humid places using the SURFACE R package (Ingram & Mahler, 2013).
Results
Phylogeny: The Bayesian tree, based on mitochondria and chloroplastic sequences, we obtain the phylogenetic hypotheses for Neotropical species of Capparaceae. Capparaceae its monophyletic and includes at least two main linages, Crateva and all Neotropical species (Fig. 1). Our outcomes also show that Cynophalla and Quadrella to be monophyletic groups, and Capparidastrum and Crateva paraphyletic. Likewise, we also found that sister lineages of the neotropical species inhabited Africa and Australasia and that Crateva was the sister clade of the rest of Capparaceae. The median age divergence for neotropical lineages was 23.3 Mya and for the Crateva clade, 9.54 Mya (Fig. 2).
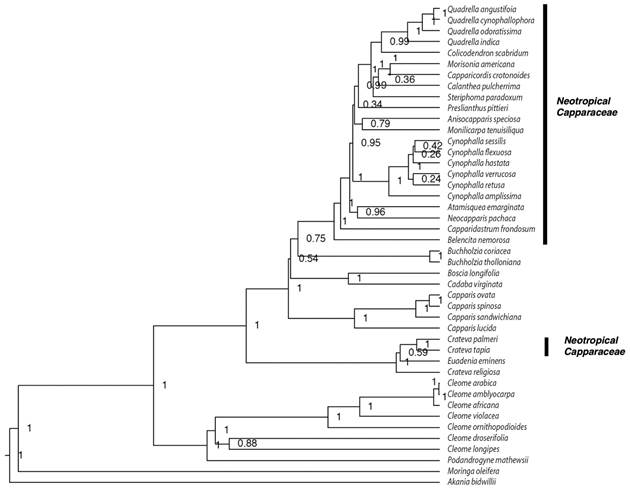
Fig. 1 Bayesian maximum clade credibility phylogenetic tree for Capparaceae based from cpDNA (ndhF, matK, rbcL) and mtDNA (rps3) sequence data. Bayesian posterior probabilities (PP) values are indicated next to the nodes.
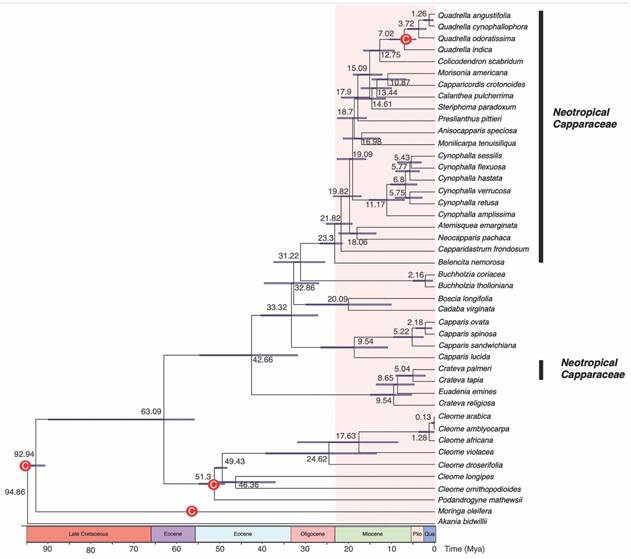
Fig. 2 Time calibrated bayesian maximum clade credibility phylogenetic tree for Capparaceae based from cpDNA (ndhF, matK, rbcL) and mtDNA (rps3) sequence data and five fossil calibrations. The values to the right of each node, indicate mean divergence times with the bar representing the 95 % highest posterior density intervals. The red nodes (C) represent the fossil (primary) and secondary calibrated nodes.
Niche quantification and comparison: According Mantel test, the values of observation (0.91) of the Pearson correlation between the niche overlap index (D) and phylogenetic distance matrices and the p-value = 0.94 indicate that our observed matrix correlation has not difference or is similar to those obtained from that resulting of a random process of scrambling the rows and column of the two matrices and, therefore, show a strong positive correlation between climatic niche and phylogeny indicating that phylogenetically more closely related species have more similar niches than species more distantly related.
The PCA-env returned two axes that together explained 72 % of the data variation, where PC1 explained 56 % and PC2 16 %. The former explained the bioclimatic variables of precipitation (BIO12, BIO16, BIO17, BIO18, and BIO19), while the latter explained the temperature (BIO4, BIO 8, and BIO9). Precipitation variables were the most important bioclimatic variables (Appendix 3). The hypotheses of niche similarity in almost all paired comparison between neotropical Capparaceae species were accepted (p ? 0.05, Table 1). In Quadrella, the niche similarity analysis showed statistically significantly niche overlap under the (Warren et al., 2008) null model (e.g. Q. cynophallophora-Q. angustifolia, D = 0.26; Q. cynophallophora-Q. indica D = 0.44; Q. cynophallophora-Q. odoratissima, D = 0.48). Similarly, we also found significant niche overlap between several genera (Morisonia-Capparicordis D = 0.68; Atamisquea-Neocapparis D = 0.24; Colicodendron - Quadrella D = 0.68). Furthermore, the climatic niche of Preslianthus pittieri (Standl.) Iltis & Cornejo was not similar to other species, while the climatic niche of Atamisquea emarginata Miers ex Hook. & Arn. was similar to three species (p ≥ 0.05; Table 1). Conversely, Capparicordis crotonoides (Kunth) Iltis & Cornejo, Colicodendron scabridum (Kunth) Seem, Cynophalla hastata (Jacq.) J. Presl, and Cynophalla flexuosa (L.) J. Presl showed that their climatic niches were more similar than expected by chance (Table 1).
Table 1 Niche similarities of the studied taxa based on the Schoener’s D Asterisks (*) indicate significant results (P < 0.05).
| D/I | Anisocapparis speciosa | Atamisquea emarginata | Belencita nemorosa | Calanthea stenosepala | Capparicordis crotonoides | Capparidastrum frondosum | Colicodendron scabridum | Crateva palmeri | Crateva tapia | Cynophalla amplissima | Cynophalla flexuosa | Cynophalla hastata | Cynophalla retusa | Cynophalla sessilis | Cynophalla verrucosa | Monilicarpa tenuisiliqua | Morisonia americana | Neocapparis pachaca | Preslianthus pittieri | Quadrella angustifolia | Quadrella cynophallophora | Quadrella indica | Quadrella odoratissima |
| Atamisquea emarginata | 0.38 | ||||||||||||||||||||||
| Belencita nemorosa | 0.30 | 0.00 | |||||||||||||||||||||
| Calanthea stenosepala | 0.22 | 0.00 | 0.42 | ||||||||||||||||||||
| Capparicordis crotonoides | 0.59 | 0.38 | 0.49 | 0.033 | |||||||||||||||||||
| Capparidastrum frondosum | 0.13 | 0.16 | 0.52 | 0.22 | 0.45 | ||||||||||||||||||
| Colicodendron scabridum | 0.58 | 0.20 | 0.49 | 0.09 | 0.63 | 0.72 | |||||||||||||||||
| Crateva palmeri | 0.35 | 0.12 | 0.48 | 0.19 | 0.70 | 0.72 | 0.43 | ||||||||||||||||
| Crateva tapia | 0.10 | 0.18 | 0.42 | 0.23 | 0.44 | 0.81 | 0.28 | 0.61 | |||||||||||||||
| Cynophalla amplissima | 0.06 | 0.02 | 0.096 | 0.08 | 0.39 | 0.55 | 0.49 | 0.47 | 0.22 | ||||||||||||||
| Cynophalla flexuosa | 0.52 | 0.22 | 0.48 | 0.19 | 0.68 | 0.48 | 0.62 | 0.36 | 0.82 | 0.46 | |||||||||||||
| Cynophalla hastata | 0.48 | 0.33 | 0.40 | 0.17 | 0.61 | 0.42 | 0.47 | 0.42 | 0.51 | 0.17 | 0.50 | ||||||||||||
| Cynophalla retusa | 0.40 | 0.29 | 0.27 | 0.00 | 0.61 | 0.28 | 0.62 | 0.40 | 0.39 | 0.16 | 0.55 | 0.46 | |||||||||||
| Cynophalla sessilis | 0.29 | 0.03 | 0.75 | 0.32 | 0.53 | 0.60 | 0.38 | 0.45 | 0.30 | 0.32 | 0.52 | 0.54 | 0.13 | ||||||||||
| Cynophalla verrucosa | 0.21 | 2.22 | 0.60 | 0.17 | 0.64 | 0.52 | 0.49 | 0.50 | 0.43 | 0.28 | 0.48 | 0.57 | 0.11 | 0.75 | |||||||||
| Monilicarpa tenuisiliqua | 0.21 | 0.02 | 0.57 | 0.19 | 0.64 | 0.52 | 0.47 | 0.52 | 0.61 | 0.52 | 0.53 | 0.54 | 0.24 | 0.58 | 0.82 | ||||||||
| Morisonia americana | 0.36 | 0.06 | 0.74 | 0.18 | 0.68 | 0.78 | 0.61 | 0.32 | 0.64 | 0.53 | 0.78 | 0.68 | 0.49 | 0.56 | 0.53 | 0.50 | |||||||
| Neocapparis pachaca | 0.24 | 0.00 | 0.76 | 0.30 | 0.72 | 0.61 | 0.51 | 0.10 | 0.56 | 0.36 | 0.41 | 0.55 | 0.06 | 0.67 | 0.76 | 0.50 | 0.35 | ||||||
| Preslianthus pittieri | 0.01 | 0.00 | 0.037 | 0.00 | 0.005 | 0.19 | 0.027 | 0.54 | 0.12 | 0.10 | 0.12 | 0.016 | 0.065 | 0.10 | 0.11 | 0.16 | 0.18 | 0.02 | |||||
| Quadrella angustifolia | 0.00 | 0.01 | 0.36 | 0.00 | 0.46 | 0.10 | 0.022 | 0.52 | 0.34 | 0.15 | 0.29 | 0.44 | 0.029 | 0.27 | 0.35 | 0.22 | 0.53 | 0.33 | 0.00 | ||||
| Quadrella cynophallophora | 0.38 | 0.17 | 0.31 | 0.37 | 0.63 | 0.40 | 0.39 | 0.54 | 0.32 | 0.20 | 0.51 | 0.57 | 0.55 | 0.42 | 0.47 | 0.55 | 0.51 | 0.40 | 0.018 | 0.26 | |||
| Quadrella indica | 0.36 | 0.01 | 0.62 | 0.29 | 0.55 | 0.40 | 0.64 | 0.52 | 0.68 | 0.73 | 0.68 | 0.63 | 0.31 | 0.50 | 0.70 | 0.67 | 0.84 | 0.77 | 0.15 | 0.44 | 0.53 | ||
| Quadrella odoratissima | 0.38 | 0.18 | 0.45 | 0.22 | 0.67 | 0.75 | 0.58 | 0.35 | 0.64 | 0.42 | 0.47 | 0.54 | 0.22 | 0.41 | 0.47 | 0.66 | 0.82 | 0.64 | 0.08 | 0.48 | 0.55 | 0.54 | |
| Steriphoma paradoxum | 0.44 | 0.34 | 0.24 | 0.006 | 0.38 | 0.47 | 0.42 | 0.55 | 0.40 | 0.17 | 0.28 | 0.25 | 0.090 | 0.18 | 0.48 | 0.62 | 0.46 | 0.38 | 0.22 | 0.10 | 0.24 | 0.50 | 0.48 |
Niche evolution analyses: We found that temperature seasonality (BIO4), mean temperatures of wettest (BIO8) and driest (BIO9) quarters, annual precipitation (BIO12), and precipitations of the wettest (BIO16), the driest (BIO17), the warmest (BIO18) and coldest (BIO19) quarters, have phylogenetic independence (λ near to 0) or low phylogenetic signals. According to the mode of evolution (κ), all climatic variables analyzed here, excluding precipitation during the coldest quarter (BIO19), indicated proportionally more evolutionary change in shorter branches and, therefore, suggesting punctuated equilibrium (κ = 0). In addition, we found that longer paths contributed more to the evolution of climatic variables that represent the realized niche of the Capparaceae (δ = 1) and, hence, species-specific adaptation has been dominant throughout the evolutionary history of this family. However, strong phylogenetic signal (λ = 1) and gradual evolution (κ = 1) were found in the precipitation of the coldest quarter (BIO19). The WN evolutionary model had a better fit in BIO4, BIO8, BIO9, BIO12, BIO16, BIO 17 and BIO18, while BM had a better fit in BIO19 (Table 2).
Table 2 Outcomes of λ, κ and δ parameters and AICc for evolutionary models
| Models | BIO4 | BIO8 | BIO9 | BIO12 | BIO16 | BIO17 | BIO18 | BIO19 |
| Lambda | 1.68E-144 | 2.39E-112 | 2.88E-203 | 2.37E-203 | 5.58E-89 | 3.46E-132 | 3.79E-181 | 1* |
| Kappa | 2.04E-187 | 1.98E-179 | 1.66E-171 | 1.06E-136 | 9.40E-02 | 6.74E-201 | 1* | |
| Delta | 2.9 | 2.9 | 2.9 | 2.9 | 2.9 | 2.9 | 2.9 | 1.59* |
| BM | 318.49 | 171.78 | 128.86 | 456.83 | 397.05 | 365.63 | 391.13 | 318.92* |
| OU | 294.34 | 144.05 | 108.04 | 444.59 | 380.66 | 354.29 | 369.53 | 320.87 |
| EB | 321.12 | 174.41 | 131.49 | 459.46 | 399.68 | 368.26 | 393.76 | 321.55 |
| WN | 291.69* | 141.40* | 105.40* | 442.08* | 378.26* | 353.36* | 366.90* | 330.28 |
| Drift | 321.12 | 174.41 | 131.49 | 459.46 | 399.68 | 368.26 | 393.76 | 321.55 |
Brownian Motion (BM), Ornstein-Uhlenbeck (OU), Early Burst (EB), White Noise (WN) and Drift Models (DF). Asterisks (*) indicate the best fits models.
The ancestral climatic niche reconstruction for the precipitation of the coldest quarter (which had a strong phylogenetic signal) was represented by a color gradient over a simplified phylogenetic tree (Fig. 3A). This suggests that the most recent common ancestor (MRCA) of the Neotropical Capparaceae evolved toward seasonal drought during coldest periods. We found that the MRCA of Crateva was inferred to have occupied sites with medium values of precipitation during the coldest quarter (Fig. 3A) at around 500 mm. Further, the MRCA of the other Neotropical species evolved from places with humidity around 300 mm during seasonal drought periods, occurring in both humid and dry forest. P. pittieri and C. frondosum evolved towards more humid places, but their MRCA occurred in drier areas. According to our results, the MRCA of P. pittieri inhabited drier places but then one clade evolved towards humid areas and another towards dry forests, including the clades Quadrella, Morisonia, Calanthea and Capparicordis (Fig. 3A).
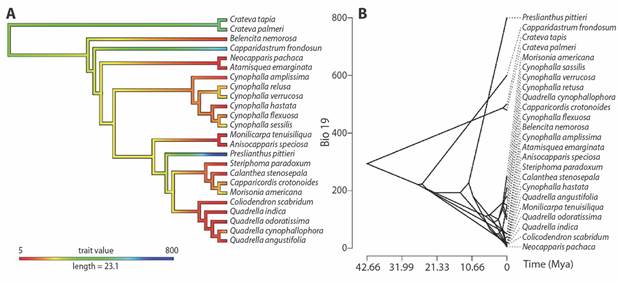
Fig. 3 Ancestral reconstructions for precipitation of coldest quarter, BIO19. A. Ancestral character reconstructions. Colours of branches reflect values of climatic variables scores estimated by maximum likelihood on the MCCT. B. Ancestral state reconstructions. X-axis represents divergence times (Mya) and the y-axis represents the reconstructed mean character values.
According to the phyloclimatic reconstruction (Fig. 3B), we found that the Neotropical species of Capparaceae shifted towards humid and dry areas independently in several lineages. It is noteworthy that ancestral character reconstructions clearly show that all Neotropical clades had a more pronounced evolution of climatic niches closer to the present time, particularly in the last 10 Mya. These outcomes are supported by the convergent evolutionary analysis (Fig. 4A, Fig. 4B) that revealed two climatic regimes: niche convergence towards dry (C. scabridum and A. emarginata ancestor) and niche convergence towards humid environments (P. pittieri ancestor) (Fig. 3B). Similarly, evolutionary rates were higher for precipitation (73 983.96) than they were for temperature (15.09) across the evolution of the species analyzed here.
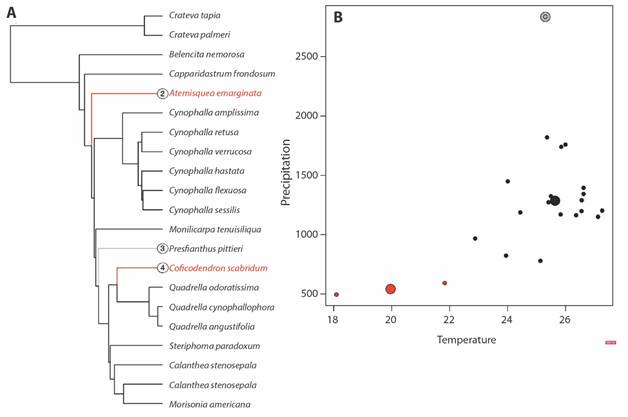
Fig. 4 Convergent evolution of climatic niche in Neotropical Capparaceae using a SURFACE approach. A. Calibrated phylogeny for Neotropical Capparaceae species with climatic niche data and adaptive regimes identified. Coloured branches represent convergent adaptive peaks (or climate regimes) and grey or black branches represent non-convergent regimes. B. Climatic space occupied by species analyzed, large points represent the same convergent adaptive regimes identified in the phylogeny and small points represent the species that have evolved around these adaptive optima. Red points represent convergent regimes toward dry forest, while grey point to humid forest.
Discussion
The results of this study provide a comprehensive view of how some species of Capparaceae evolved through the climatic space through their evolutionary history. We evaluated relationships between climatic niche attributes and phylogenetic relationships with the aim of establishing the climatic niche dynamics of this family when colonizing dry forested areas in the Neotropics. We found some broad climatic affinities (niche overlap) among Capparaceae species using a multivariate analysis of climatic variables. Besides we found niche overlap within Quadrella species and between Morisonia-Capparicordis, Atamisquea-Neocapparis and Colicodendron-Quadrella we also found significant relationships between the climatic niche overlap index (D) and phylogenetic distance (Mantel test; P = 0.94). However, this latter outcome was found at genus level.
Although we did not find a strong phylogenetic signal in most climatic variables herein analyzed and apparently PCND seems likely to play a major role in the evolution of Capparaceae, we found that precipitation of the coldest quarter (BIO19) had a strong phylogenetic signal (λ), and a slow (κ) and gradual evolution (δ) were better supported, while Brownian Motion was the best fitted evolutionary model. According to Crisp and Cook (2012) and Münkemüller et al. (2015) these results could be considered as consistent with a scenario of PNC for adaptations to precipitation of the coldest periods. Coldest periods mainly include the months December, January, February, and March. Although the frequency and intensity of seasonal drought depends largely upon latitudinal position (Arango et al., 2021), with the shortest and least severe dry periods found on or within few degrees of the equator (Murphy & Lugo, 1986), it is clear that all NSDF fragments have a prominent dry period during the coldest months (Gentry, 1995), which could even drive the phylogenetic information into communities along latitudinal gradients (Arango et al., 2021). Drought stress tolerance is one the most important adaptations of NSDF species to resist seasonal long-term drought (Engelbrecht & Kursar, 2003). The latter can be defined as the ability of species to survive desiccation while minimizing reductions in growth and fitness (Engelbrecht & Kursar, 2003). We suggest that colonization of Capparaceae to new dry areas occurred via environmental filtering that produced phylogenetic niche conservatism of those traits related to drought stress. This pattern has been found in other clades of dry forest (Angulo et al., 2012; De-Nova et al., 2012).
Likewise, we found that the realized niche evolution of Capparaceae was more concentrated in derived phylogenetic branches (e.g. recent evolutionary influence traits) than in deep phylogenetic splits (Cooper et al., 2010) and, therefore, that lability was intimately connected to their expansion into new climate space at least 10 Mya (Fig. 3A) ago and the more recent evolution toward the dry and humid forest. Neotropical species of this family evolved from ancestors that inhabited humid sites, where e.g. Crateva, the sister clade of all neotropical species inherited this condition from their ancestors and currently occurs at humid sites (Mercado-Gómez et al., 2020). However, during the Middle Miocene species-specific adaptations to drier sites also occurred throughout an arid period, which was further enhanced by a global cooling phase that resulted in polar ice sheets with the addition of concomitant local volcanism might have enhanced rain shadows due to temperature drop (Churchill & Linares, 1995; Hernández-Hernández et al., 2013; Hernández-Hernández et al., 2014).
Moore and Robertson (2014) suggested that ecological opportunities emerged starting from long-distance dispersal events, when an alien lineage took advantage of an array of newly formed or previously unfilled niches and particularly when a change in the environment made new resources available. This would have created new challenges or opened new environmental niches (Tan et al., 2016). Within the Miocene and Pliocene, new niches emerged from the cooler/drier conditions that produced drought-stress, and this presented strong selective pressures on lineages to evolve and successfully survive and reproduce in these new environments (Hernández-Hernández et al., 2014). Niche availability, understood as an ecological opportunity (Michel et al., 2013; Stroud & Losos, 2016), also may affect the species diversification dynamics and therefore plays an important role in species evolution (Paun et al., 2016). It is very likely that species of Capparaceae might have responded to Miocene aridification adapting to the drier conditions that emerged as a novel ecological space (Hernández-Hernández et al., 2014).
We detected convergent and asychronically evolution in Capparaceae to dry places during the driest periods of the Miocene (Churchill & Linares, 1995; Hernández-Hernández et al., 2013; Hernández-Hernández et al., 2014) in B. nemorosa (23 Mya), Neocapparis-Atamisquea (18 Mya), Monilicarpa-Anisocapparis (16 Mya), Calanthea-Morisonia-Capparicordis (14 Mya), Colicodendron (12 Mya) and Cynophalla (11 Mya). Our results were consistent with those found by De-Nova et al. (2012) in Burseraceae, who find that during these arid periods, species of this family adapted and expanded their distributions from Mexico to South America. Furthermore, Willis et al. (2014) also found similar results in the Malphigiaceae, and Hernández-Hernández et al. (2014) suggested that the radiation of the Cactaceae coincided with the expansion of aridity in North America during the late Miocene. An increase of diversification during these arid periods is also consistent with the NSDF that arose in North America during the middle Eocene and expanded in distribution southwards towards Central and South America throughout the Miocene and have persisted for 19 Ma hosting endemic species that exhibiting strong phylogenetic niche conservatism (Lavin, 2006; Pennington et al., 2004; Pennington et al., 2009; Särkinen et al., 2012).
Although we found evidence of PNC in the precipitation of the coolest quarter variable (BIO19), an overall climatic differentiation and convergent evolution was found in both, species adapted to dry and species adapted to humid forests. Ecological convergent evolution was found at least two clades (Atamisquea - Cynophalla and Colicodendron - Quadrella), such clade-wide convergence can be interpreted as lineages independently responding to the same selective regimes, or equivalently, discovering the same adaptive peaks on a macroevolutionary adaptive landscape (Ingram & Mahler, 2013), suggesting that niche conservatism has not played the major role in the early evolution of the Neotropical species of this family. Our outcomes show that PNC possibly played a major role during the late evolution of the group, where it can be detected in some clades like Cynophalla and Quadrella mainly. Angulo (2012), found in Barkeria, that this clade shows PNC to dry forest since the middle Pliocene to the Pleistocene. However, our phylogenetic bias (only 24 of species included in the phylogeny) does not allow us to better understand the evolution of Neotropical Capparaceae and therefore more analyses are necessary to improve the knowledge of the climatic history of this important dry forest family.
Our results also support that high heterogeneity and patchy distribution of habitat resulting from climatic changes during the Miocene have allowed convergent evolution towards humid sites in Capparidastrum and Preslianthus (Fig. 3A, Fig. 3B). Nevertheless, this evolution occurred independently multiple times. Preslianthus (e.g. evolved into humid places during the middle Miocene) but C. frondosum evolved into humid sites later in the Pliocene. However, our outcomes also showed that their MRCA (Fig. 3B) was adapted to dry areas. Mercado-Gómez et al. (2020) found that C. frondosum and C. tapia have larger climatic variations correlated with greater distribution areas for species, a correlation known as niche breadth. Niche breadth explains why these species are tolerant to dry and moist environments along the transitional areas between ecosystem (Sexton et al., 2017).
Analyzing climatic niche evolution among species of Capparaceae based on environmental data and phylogenetic information, we found that the Neotropical species of this family may have emerged from the Middle Miocene, where its ancestor inhabited humid sites. We also found that during the driest periods of the Miocene, both divergent and convergent evolution occurred, resulting in two main groups associated with dry and humid forests.
Our analysis recovered the evolutionary history of the climatic niche of the Capparaceae toward humid and dry places. Furthermore, taxa constrained by NSDF also show convergent evolution more than phylogenetic niche conservatism. However, due to our sparse phylogenetic sampling (24 species from 104), our findings only allow us to suggest that Cynophalla and Quadrella are the only taxa that might provide robust inferences about the NSDF flora evolution. We suggest more detailed phylogenetic analyses including more Neotropical taxa and additional morphological data such as leaves or woody traits in order to search for additional evidence that might improve our knowledge on the evolutionary history of NSDF and the evolution of Capparaceae in this biome.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgments section. A signed document has been filed in the journal archives.
See Digital Appendix - a10v70n1-s1











 uBio
uBio 


