Lytechinus A. Agassiz is a echinoid genus of the Americas with species on both coasts. L. variegatus (Lamarck) inhabits the coastal waters from North Carolina (USA), throughout the Gulf of Mexico to southern Brazil (Lawrence, 2001). Its populations inhabit a wide variety of shallow sub littoral habitats including sea grass beds, on sub tidal hard bottoms covered or not with algae, rock, shell hash or sand (Junqueira et al., 1997; Beddingfield & McClintock 2000; Lawrence, 2001).
Abiotic factors as temperature and salinity were more important in regulating population dynamics of this species. In most shallow water habitats densities of L. variegatus can vary, both seasonally and annually. The absence of L. variegatus in an appropriate habitat may be the result of episodic biotic or abiotic factors, particularly in nearshore populations (Junqueira et al., 1997; Beddingfield & McClintock, 2000).
As a ruderal species, L. variegatus exhibits rapid growth, early reproductive maturity and short longevity are important life history adaptations. Gonochoric individuals began to reproduce at approximately 40 mm diameter at an age of one year (Moore et al., 1963; Lawrence & Bazhin, 1998; Beddingfield & McClintock, 2000). Spawning events is generally seasonal, but reports of the timing and duration of reproductive patterns on its populations from different geographic areas vary widely, indicating a habitat-dependent both local than latitudinal (Ernest & Blake, 1981; Lessios, 1985).
This paper examines the reproductive cycle of Lytechinus variegatus near the southern limit of its geographic distribution throughout gonad index and histological analysis of the gonad tissue.
Materials and methods
The present study was conducted from September 2000 through September 2001 in Paranaguá Bay (25° 20 - 26´ S and 48° 05 - 36´ W), southern Brazil. Specimens were collected from an unvegetated intertidal flat with muddy/ sandy sediment situated in the eurihaline region of the estuary. Seawater temperatures exhibited a seasonal pattern from 19 to 28 oC. Due to a well-defined saline stratification, the middle section of the estuary shows a small range of salinity parameter from 25 to 30. Summer values around 20 predominate due to the increase in fresh water inflow and rain regime (Machado et al., 1997).
Sea urchins with 50.8 to 77.0 mm test diameter size range were collected at low tide from shallow bottoms. They were preserved in saline formalin then transferred to 70 % ethanol for histological examination. All gonads were removed and four of five were dried to 60 °C to constant weight and weighted to 0.00001 g. Gonad index [(gonad weight/total body weight)*100)] was calculated for both sexes. One preserved gonad was dehydrated, embedded in paraffin wax, placed in marked paraffin blocks and cut with a microtome to 7 µm sections. Slides were stained with hematoxylin and eosin and cover slips were mounted using Permount.
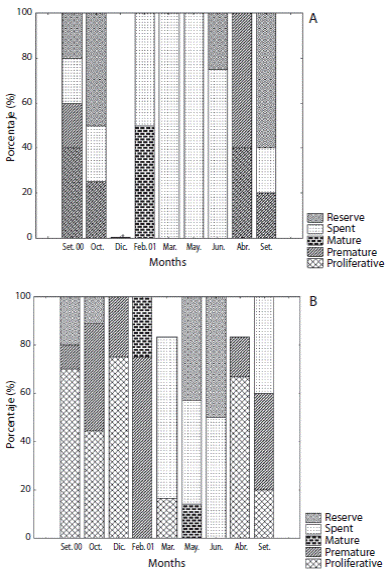
The gonad development of each individual was established in accordance with gametogenic stages described by Tavares and Borzone (2006) and their frequencies were plotted by sex. Sex ratio was tested using Chi-square (χ2) test. Biometrics data as oocyte diameter was measured by the long axis of each cell and only the oocytes with a visible germinal vesicle were considered. From these data, oocyte diameter frequency distributions were plotted. Spermatogenic tubule selected sections were examined to estimate the amount of sperm present by measuring the cross-sectional area of sperm and nutritive phagocytes relative to the total cross-sectional area. Statistical differences were tested between samples by one-factor ANOvA for gonad index and relative rate of nutritive tissue/male gametes. Differences were considered statistically significant at p < 0.05.
Results
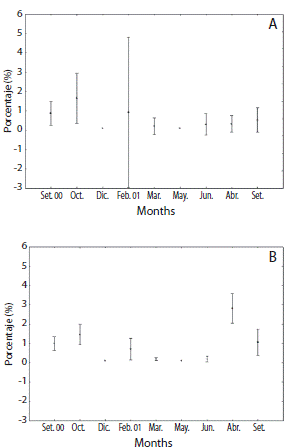
Eighty six individuals (56 males and 30 females) were identified during eight sampling months. Population showed no statistical difference from 1:1 sex ratio (χ2 = 2.57; DF = 7; p < 0.05). Females gonad index (GI) varied from 0.21 to 1.64 (F = 3.54; p < 0.05) and GI males from 0.19 to 2.80 (F = 12.86; p < 0.0000) (Fig. 1). Both sexes showed similar trends with a first peak in October 2000 and subsequent marked decline on the index from February to July 2001. Gonads began to increase in size in August 2001 but only for males a bimodal pattern was more evident.
Female reproductive cycle showed half part of individuals at resting and proliferative stages during September and October 2000 (Figs. 2 and 4A). Mature stages were observed mainly at February 2001. Spawning was pronounced at March and July 2001 when almost all females were at spent stage. An advanced gametogenic activity was observed during August and September 2001 with a quick progression of nutrient accumulation, oocyte production and spawning events in a brief period. Males showed an intense proliferative stage and maturing period from September 2000 to February 2001 (Fig. 3 and 4B). A marked spawning also was observed in March and July 2001.
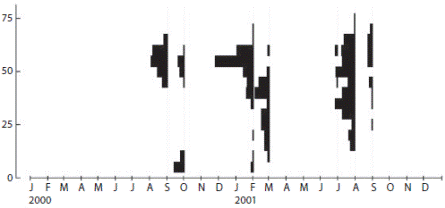
The oocytes size distributions ranging between 6.8 and 75.6 μm in diameter (Fig. 5). Oocytes 50 to 60 μm were typically present in the gonads year round. September 2000/2001 and February 2001 distributions (spring and summer months) generally had larger ranges of oocytes of 70 to 75 μm in diameter. Oocytes from the October sample were small (< 14 μm) when there was evidenced a new cohort of primary oocytes.
Biometrical analysis in testis showed an inverse relationship between relative quantities of nutritive tissue and the area occupied by mature spermatozoa (Figs. 6A and 6B). Male gonads exhibited a nutritive phagocytes meshwork, which ranged significantly from 14.7 (February 2001) to 74.7 % to the total crosssectional area (September 2000) (F = 47.7; p < 0.0000). Percentage of mature spermatozoa varied significantly from 2.6% (July 2001) to 63.9 % (February 2001) (F = 21.5; p < 0.0000). The amount of sperm increased once again to 50.2 % and nutritive phagocytes also reduced to 21.1 % in September 2001.
Discussion
Reproduction of L. variegatus in southern Brazil exhibits an annual cycle of gonad development. Synchronicity on maturation and spawning periods could be observed in summer and fall months. Marked reproductive seasons have also been noted among its populations in the northern limit of this species although mature and spent individuals could be found at any time of the year. Spawning events were observed more intense once (Ernest & Blake, 1981) and twice a year (Beddingfield & McClintock, 2000) or same with a lunar rhythm (Moore et al., 1963). Timing of the peak spawning season could be varying from year to year as observed by Moore and Lopez (1972).
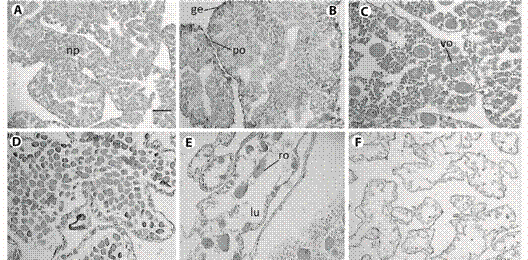
Fig. 2 Histological sections from female gonad of L. variegatus. A: Resting: oogenic tubules were filled by nutritive phagocytes; B: Proliferative: development of oocytes in the germinal epithelium and depletion of nutritive phagocytes; C: Premature: previtellogenic oocytes increasing in size; D: Mature: vitellogenic oocytes filling the lumen; E: Spent: tubules showed few relict oocytes, phagocitosis is evident; F: Late spent individual. Any cell was found in the vacated lumen. np = nutritive phagocytes, ge = germinal epithelium po = previtellogenic oocyte, vo = vitellogenic oocyte, ro = relict oocyte, lu = lumen. Scale bar: 50 µm.
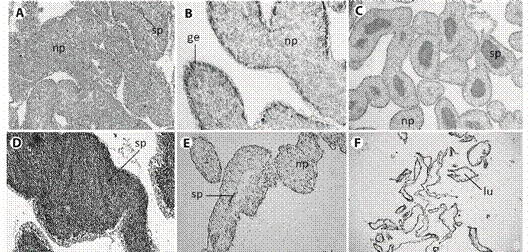
Fig. 3 Histological sections from male gonad of L. variegatus. A: Resting. spermatogenic tubules were filled by nutritive phagocytes, some amount of spermatozoa can occur; B: Proliferative. Nutritive phagocytes filling the lumen, germinal epithelium can be observed; C: Premature: spermatozoa quantity x depletion of nutritive phagocytes increasing; D: Mature: maximum concentration of spermatozoa; E: Spent:. few spermatozoa occupies a reduced lumen; F: Late spent individual: advanced lysing of tissue. np = nutritive phagocytes, ge = germinal epithelium, sp = spermatozoa, lu = lumen. Scale bar = 50 µm.
These episodic events may be important in regulating aspects of L. variegatus population dynamic, mainly when size-specific death occur, as the significant loss of juveniles, less resistant to dramatic changes of abiotic factors (Beddingfield & McClintock, 1994; Junqueira et al., 1997). Lytechinus variegatus population in Paranaguá Bay was characterized by a few numbers of adult individuals sizes with a sparsely distribution. Several works reported abiotic factors regulating abrupt changes in its densities. Temperature, salinity or combinations of both variables were responsible for mass mortality throughout its range distribution (Moore et al., 1963; Lawrence, 1975; Beddingfield & McClintock, 1994; Junqueira et al., 1997). The present study area is a complex environment, strongly controlled by physical and climatic factors, which affect the benthic community. Marked dilution by freshwater discharges during summer rains, strong tide currents and intensive solar exposition due to the shallowness of the water, results in extreme conditions, which could be affect size structure of this sea urchin population (Lana et al., 2001).
Evaluations of L. variegatus reproductive patterns revealed a high reproductive capacity throughout its range distribution. According to Lessios (1985) in strongly seasonal environments the fluctuation of physical parameters may impose rigid selective regimes, which controlling the mode and timing of reproduction. On some Caribbean echinoids reproductive pattern was considered less consistent due to the ripe condition of the majority of the population throughout the year (Lessios, 1985; Cameron, 1986; Lessios, 1991). At the southeast of Brazil (Cabo Frio and Ribeira Bay regions) Junqueira et al. (1997) also indicated a non remarkable reproductive cycle in both populations analyzed.
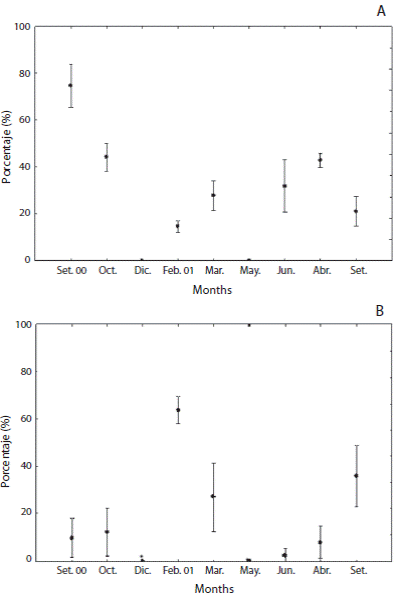
Fig. 6 Relative amounts of somatic and germinative tissue of L.variegatus. A: nutritive phagocytes; B: spermatozoa.
Reproductive patterns in sea urchins involve sometimes nutrient accumulation into nutritive phagocytes. Typically, gonads increase in volume in spring and sometimes again to lesser extent in late summer or fall (Ernest & Blake, 1981; Cameron, 1986, Beddingfield & McClintock, 2000). For some authors, food resources act as primary factors in determining variability in resource allocation. Type and quality of food can also affect gonad development (Bishop & Watts, 1994; Beddingfield & McClintock, 1998; Junqueira, 1998; Hammel et al., 2004). Most of gonad measurements of L. variegatus as gonad index (GI) may be strongly influenced by nutrients accumulation and the increases in gonad size not clearly reflects gonad maturity (Ernest & Blake, 1981; Bishop & Watts, 1994).
In the present study the higher gonad index (GI) during the winter and spring were closely aligned with the initial gametogenic activities as resource allocation and growing of gametes. At Rio de Janeiro populations (southeastern Brazil) Junqueira (1998) also found few correlations between GI and gametogenic cycle since gonad increment were correlated with resting, proliferative and maturation stages.
Sex-specific differences in both gonad growth and gamete development were already observed in L. variegatus populations. In general large rates of ovarian growth were explained by more energy being allocated to ovaries which require large amount of nutrients to produce its gametes. In relation to gamete production, sperm are quickly produced with relatively little energy, whereas the process of increasing oocyte size takes much longer periods of time to reach gamete maturity (Pearse & Cameron, 1991; Bishop & Watts, 1994).
Many works have examined differences between maximal ovarian and testicular indices. Moore et al. (1963), Bishop and Watts (1994) and Junqueira (1998) observed that females attained an equal or higher index than males meanwhile Moore and McPherson (1965) found no differences. In our study low female index values might be explained by the constancy of spent individuals. The presence of males in proliferative stage during most part of year corroborated an existence of a continuous production of gametes.
Biometrical analysis on testis showed an inverse relationship between nutritive somatic tissue and mature spermatozoa throughout spermatogenic cycle with a massive presence of mature spermatozoa in summer. Competitive interaction between phagocytic and gametogenic cycles has been demonstrated for echinoids and appears to be a universal process (Ernest & Blake, 1981) As males, females showed more investment in number and mainly size gametes also in summer season.
Ooocyte dimension is another parameter related to the mode of development and allocation of resources in echinoderms (Lessios, 1987). Mature oocyte found for L. variegatus (60 μm average) is the highest oocyte size developed by sea urchin species in the same latitude, such as Echinometra lucunter Linnaeus, 1758 (48 µm average) and Arbacia lixula Linnaeus, 1758 (35 µm average) (Tavares, 2004). Some intraspecific variation may be occurring when local population is compared. In fact, subtropical Brazilian populations of L. variegatus showed an intermediate mature oocyte size in relation to both temperate populations where oocyte average range about 50 µm (Ernest & Blake, 1981) and 90 µm (Bishop & Watts, 1994). Our results suggest that gametogenesis in this echinoid could be modulated by local environment factors and play predominant role in the timing and magnitude of reproduction in populations of L. variegatus.












 uBio
uBio