Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.59 n.1 San José Mar. 2011
In vitro rhizogenesis: histoanatomy of Cedrela odorata (Meliaceae) microcuttings
Liliana Millán-Orozco, Elena Corredoira & Maria del Carmen San José
Laboratory of Plant Physiology, Instituto de Investigaciones Agrobiológicas de Galicia, CSIC, Avda. de Vigo s/n, 15705 Santiago de Compostela, Spain; lmilln@yahoo.com, elenac@iiag.csic.es, sanjose@iiag.csic.es
Dirección para correspondencia
Abstract
Cedrela odorata (Meliaceae) is considered as one of the most valuable forest tree in the tropics. Clonal propagation of this species provide an alternative method to propagate superior genotypes, being the production of good quality adventitious roots one of the most important steps in micropropagation techniques. The sequence of anatomical changes that takes place during the formation of adventitious roots in shoots of Cedrela odorata cultured in vitro is described in this study. Eigth-week-old shoots, from multiplication cultures, were rooted in Murashige and Skoog´s medium (1962) with half- strength macronutrients and with 0 or 1mg/l indole-3-butyric acid (IBA). Between 12 and 24h after the start of rooting, some cambium, phloem and interfascicular parenchyma cells became dense cytoplasm, nuclei with prominent nucleoli and the first cell divisions were observed, especially in shoots treated with auxin (dedifferentiation phase). After 3-4 days, the number of dedifferentiated cells and mitotic divisions increased considerably, and the formation of groups of some 30-40 meristematic cells (meristemoids) was observed (induction phase). The first primordial roots developed from the 4th-5th day. The vascular tissues of these primordia connected to those of the explant, and roots began to emerge from the base by day 6. Development of the primordial roots was similar in the control shoots and shoots treated with 1mg/l IBA, although there were more roots per explant in the latter. Rev. Biol. Trop. 59 (1): 447-453. Epub 2011 March 01.
Key words: in vitro culture, adventitious rooting, anatomical study, Spanish cedar, histology.
Resumen
Cedrela odorata (Meliaceae) es una especie tropical de gran valor económico. La propagación in vitro de esta especie ofrece una vía alternativa para la clonación de genotipos superiores, siendo la formación de un buen sistema radical uno de los pasos claves en la micropropagación. En este trabajo analizamos la secuencia de cambios anatómicos que tienen lugar durante la formación de raíces adventicias en microestaquillas de Cedrela odorata. Para el enraizamiento se utilizó el medio MS con los macronutrientes reducidos a la mitad, suplementado con AIB 0 ó 1mg/l. A partir de las 12-24 horas del comienzo del enraizamiento, se observaron los primeros cambios en las células del cambium, del floema y del parénquima interfascicular (fase de diferenciación). Después de 3-4 días, aparecen grupos de células meristemáticas (fase de inducción). Los primordios se desarrollan después de 4-5 días, siendo visibles al exterior a partir del sexto día (fase de emergencia). El desarrollo de las raíces fue similar en ambos tratamientos, pero la presencia de AIB aumenta el número de raíces.
Palabras clave: cultivo in vitro, enraizaimiento adventicio, estudio anatómico, cedro, histología.
Species of the genus Cedrela (Sapindales: Meliaceae) are widely distributed across the American continent, and form part of the autochthonous flora in all South American countries from Mexico to Argentina (except Chile) and also in the Antilles (Betancourt 1999). Of the seven species that make up the genus, Cedrela odorata L. (cedro) is considered as one of the most valuable forest tree species in the tropics and also one of the most widely distributed. Cedro wood is greatly valued for its quality, its ductility and durability, and is used to make fine furniture and decorative veneer. Moreover, some compounds extracted from the bark and leaves of this species has been shown to display antimalarial activity (Omar et al. 2003).
Natural cedro is propagated from seed in many parts of Central and South America, but good initial growth is often followed by dieback after 2-3 years. Moreover, cedro does not coppice readily nor produce root suckers. These features, combined with attack from Hypsipyla grandella Seller (Lepidoptera, Pyralidae) and the continual selective exploitation to which many species of the genus Cedrela is subjected, especially C. odorata, have led to serious deterioration of the species, which has been included by the International Union for Conservation of Nature (IUCN) on their list of threatened species. Deforestation in Colombia has reached levels of 600 000 ha/year, which signified the felling of 37.7 million trees between 1960 and 1984 (PAFC 1997).
Vegetative or clonal propagation offers an alternative method to propagate superior genotypes. The application of tissue culture on mass clonal propagation of plants is the widest utilization of this technology (Thorpe 2006). However, to date very few reports have been published on in vitro culture of C. odorata (Maruyama et al. 1989, Ishii & Maruyama 1992, Cerdas et al. 1998) and reproducible protocols have not yet been established. Success in micropropagation is dependent on the production of good quality adventitious roots, whose formation has four distinct phases namely, cell dedifferentiation, induction, root primordial development and root emergence (De Klerk et al. 1999). De Klerk (2002) established that the essential characteristics to distinguish the successive phases in the regeneration process was the response to the organogenic stimulus (i.e., to various concentrations of auxins and/ or cytokinins). In the present study, histological events leading to in vitro root formation in cedro microcuttings were examined in relation to the presence of auxin in the medium.
Materials and methods
Shoots from seeds germinated in vitro were used in this study. Cultures were maintained onto a proliferation medium consisting of Murashige and Skoog medium (MS) (Murashige & Skoog 1962) supplemented with 2mg/l BA, and were transferred to fresh medium each four weeks during the eight-week multiplication cycle (Millán-Orozco & Ballester 2006). The shoots (15-20cm) were separated and used later for rooting experiments.
The rooting medium was a MS medium (half-strength macronutrients) supplemented with 1mg/l IBA, and after 24h shoots were transferred to fresh medium without IBA. The same medium without IBA was used as control medium. All media were supplemented with 100mg/l myo- Inositol, 30g/l sucrose and 6g/l agar (Sigma A-1296). The pH of all media were adjusted to 5.6 before autoclaving at 121ºC for 20min. For rooting experiments, shoots were cultured in 300ml glass jars, containing 60ml of medium. Each jar contained six shoots and at least four jars were used per experiment.
All cultures were grown under a 16h photoperiod (provided by cool-white fluorescent lamps at a photon flux density of 50-60 μmol/m2s) with 25ºC light/20ºC dark temperatures.
Batches of nine stem pieces from Control and IBA-treated shoots were harvested daily until the emergence of root primordia. The basal 4-6mm of each selected shoot was fixed in 5:5:90 (v/v/v) formalin/glacial acetic acid/50% ethanol in vials at room temperature. The fixed material was dehydrated through a gradual n-butanol series followed by infiltration with molten paraffin. Transverse serial sections, 10µm thick, were made using a rotatory microtome. Sections were double stained with either safranin-fast green for general examination or with periodic acid-Schiff (PAS)-naphthol blue-black, which is commonly used to reveal total insoluble polysaccharides and total protein content of the cells (Feder & O´Brien 1968).
Results
We have previously shown that C. odorata has a certain rooting capacity even in the absence of auxin, although the presence of IBA in the medium favours rooting and increases the number of roots and their length; the effect increases when explants were taken from 4-12-month-old seedlings established in the greenhouse. In addition, the presence of IBA during the first 24h increases the kinetic of root formation (Millán-Orozco 2006).
At the time of excision for root initiation, the anatomy of the stem was typical of stems of dicotyledons. Typical collateral vascular bundles arranged as an almost continuous ring around the pith. Vascular cambium, constituted by 3-4 layers of flattened and vacuolated cells with peripherally located nuclei, was observed between the xylem and the phloem. The cambial derivatives that develop gradually to form the secondary elements of the xylem and the phloem were also observed. An almost continuous ring of sclerenchyma formed by fibres separated by small areas of parenchymatic cells was observed around the phloem. The cortex that surrounds the phloem is formed by large parenchymatic cells and the epidermis by two layers of rectangular cells (Fig. 1A, B).
The C. odorata shoots responded very quickly, and 12-24h after rooting began, the first changes in the cells in the basal zone of the shoots were observed. Numerous cells with a more prominent nucleus and nucleoli and that stained more intensely with safranin were observed in the zone of the cambium, phloem and the interfascicular parenchyma (Fig. 1C). Numerous mitotic divisions were also observed, with anticlinal, oblique and periclinal division planes, the latter being the most abundant. Many of these divisions are especially evident in regions adjoining leaf and in general were more abundant in the IBA treated shoots than in the controls. Numerous starch grains that were intensely stained by PAS-naphthol blue were observed. The starch content decreased gradually as development of the adventitious roots progressed. The number of mitotic divisions increased by 36-48h, as did the number of meristematic
cells, which appeared with large and intensely stained nuclei in the cambium, interfascicular parenchyma and especially in the phloem. In the shoots treated with IBA short radial rows
of three-four successive dedifferentiating cells were observed in these zones (Fig. 1D).
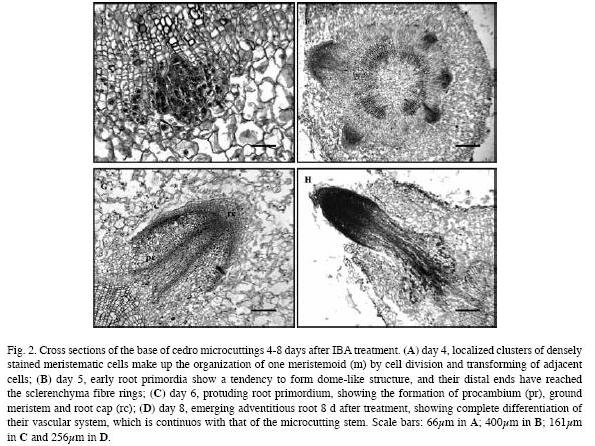
After 3-4 days there was a notable increase in the number of mitotic figures. Numerous periclinal divisions together with some oblique and anticlinal divisions were observed in cells located not only in the cambial zone, but also in the outermost layers of the phloem region. These divisions resulted in the formation of small groups of cells exhibiting meristematic
characteristics, such as isodiametric, denselystained cells with a high nucleous-to-cell area ratio (Fig. 2A). Such localized meristematic clusters develop by both periclinal and anticlinal
divisions and are assumed to make up the organization of meristemoids (Torrey 1966). More meristemoid was formed in the IBA treated shoots than in the controls. Meristemoids showed no overall polarity at this stage and appeared to originate from cambial derivatives, especially ray parenchyma cells, and they generally developed in the outermost region of phloematic tissue close to the sclerenchyma ring. The involvement of dedifferentiating cells of the surrounding inner cortex parenchyma contributing to the build-up of the meristemoid was also evident. The meristemoids were intensely stained with naphthol blue. The meristemoids continued to differentiate and at the end of 4-5 days, were forming primordial roots. These crossed the ring of sclerenchyma and were found at the level of the cortex (Fig. 2B). A certain degree of internal differentiation was observed in the primordial roots, with the procambium and the ground meristem becoming distinguishable from the apical meristem. Further division at the distal end of the apical meristem gave rise to a root cap consisting of three-four layers of cells that were more vacuolated than centrally located cells. Division continued in the cells behind the developing primordia with differentiation of tracheal elements orientated perpendicular to the vascular system of the stem and that later connected the vascular system of the stem and the emergent root (Fig. 2C). Primordial root development
was similar in both treatments, although more roots developed in the IBA treated shoots than in the controls.
Formation of the primordia is an asynchronous process, and from the 6th day, some primordial roots were observed to have crossed the epidermis and emerge to the exterior, although the greatest degree of root emergence was observed between days 8-12 (Fig. 2D), as new vascular elements connecting the vascular system of the new primordial roots to the shoot had developed by then. The pattern of root formation was the same in both treatments (control and IBA), although in the IBA treated shoots there were more explants with developed
roots, and more roots per explant.
Discussion
The success of large-scale vegetative propagation of woody and shrub plants depends on the rooting ability of the plants. In fact, success of the most hazardous step of propagation, i.e. transfer and acclimatization of in vitro rooted microcuttings to an ex vitro environment, depends on the quality of the root system. Thus, research on adventitious root formation
is highly important from the practical point of view.
In a previous study, we found that C. odorata displays a good capacity for in vitro rooting, even in the absence of auxin, although the presence of IBA in the medium was more significant as the age of the initial explants raised (from 4-12 month-old seedlings) increasing the percentage of rooting as wells as the number and length of the roots formed; root emergence was also favoured in the treated shoots (Millán-Orozco & Ballester 2006). De Klerk et al. (1999) reported that in plants in which roots regenerate spontaneously, endogenous
auxins produced at the shoot apex are transported basipetally to the cut surface and act as the trigger, removal of the apex reduces both the level of endogenous auxin in the basal portion of a cutting and the number of regenerated roots. Moreover, in these plants, application of exogenous auxin strongly increases the number of regenerated roots.
From the histological study, it is clear that C. odorata displayed the direct pattern of rhizogenesis. The present study revealed the time course of the rooting phases in microcuttings
of C. odorata and the triggering of cell division that leads to the formation of adventitious roots. The sequence of anatomical changes produced during rhizogenesis was very similar to that reported for in vitro rooting of other woody species such as apple (De Klerk 2002, Naija et al. 2008) and chestnut and oak (Ballester et al. 2009), although different species require different lengths of time for each of the stages in which the process can be divided, each with particular hormonal requirements. Root initiation involves the dedifferentiation of specific cells, leading to the formation of root meristems (Hartmann et al. 1990). In apple microcuttings, De Klerk (2002) showed that during the first days certain cells in the stem become competent to respond to the rhizogenic signal. Then, in the next days, these founder cells divide and the descendent cells become increasingly determined to root formation under the influence of the root-inducing signal (auxin). In cedro, the first evidence of root initiation is produced within 12-24h, with the appearance of cells with large nuclei and dense cytoplasm in the zone of the cambium and adjacent phloem (dedifferentiation phase). The early activation of cells by altered nuclear/ nucleolar characteristics, the altered staining properties of the cytoplasm and early cell division activity have been reported as the first cytological events leading to formation of root primordia in a number of in vitro rooted cultures (De Klerk et al. 1995, Naija et al. 2008, Ballester et al. 2009). The cells from which the root primordia originate are usually between the vascular bundles and accumulate starch during the initial 24h (De Klerk et al. 1999). As in cedro, in many studies of woody species, the site origin has been located near to or in the vascular cambium (Syros et al. 2004, Naija et al. 2008). The region of the tissue in which cells become activated is thought to depend partly on physiological gradients of substances entering the shoot from the medium and on the presence of competent cells that can respond (Ross et al. 1973).
Some 3-4 days after the initiation of rooting, we can observe groups of some 30-40 meristematic cells that may be considered as meristemoids (induction phase). Favre & Médard (1969) stated that in rooting microcuttings of Vitis the formation of "meristematic fields" (actually root meristemoids) is the essential stage of adventitious root development. In a study carried out with cuttings from juvenile and adult chestnut, Ballester et al. (1999) found that although reactivation of cell division occurred in both cases, the posterior regeneration of the cells to produce the root meristemoids only occurred in cuttings from juvenile plants. During this process the cambium, which normally functions as a secondary meristem, acquires the functional characters of a primary meristem, the apical root meristem. As in other in vitro rooting systems (Zhou et al. 1992, Ballester et al. 2009), dedifferentiated cells of the surrounding parenchyma were incorporated into the root meristemoids. After 4-5 days, the meristemoids developed into dome-shaped root primordia and then into roots (differentiation phase, De Klerk et al. 1995, 1999). Root elongation and emergence also followed the typical pattern seen in endogenous root formation. Vascular differentiation and continuity was established early and before emergence of the elongating root primordium. The tissue organization in the adventitious roots appear normal with a well-defined root cap and root apical meristem in each root.
In conclusion, the organogenic process described in this paper for C. odorata explants appears to indicate fulfilment of the determinative events defined by Thorpe (1994) for organized development in vitro: 1) A suitable inductive signal must cause changes in individual cells that become activated in an appropriate way, 2) the cells be able to undergo dedifferentitation and 3) cells must be able to interact. The same underlying mechanisms appear to operate to some extent in both shoot and root regeneration, competence for one or the other organogenic process being acquired during an initial phase of dedifferentiation (De Klerk et al. 1997).
Acknowledgments
L. Millán-Orozco is grateful to COLCIENCIAS (Colombian Institute for the Development of Science and Technology) for a grant to carry out doctoral studies abroad (IIAG, CSIC,
Santiago de Compostela, Spain). The study was partly financed by the MEC, Spain, through project AGL 2005-00709.
References
Ballester, A., M.C. San José, N. Vidal, J.L. Fernández-Lorenzo & A.M. Vieitez. 1999. Anatomical and biochemical events during in vitro rooting of microcuttings from juvenile and mature phases of chestnut. Ann. Bot 83: 619-629. [ Links ]
Ballester, A., N. Vidal & A.M. Vieitez. 2009. Developmental stages during in vitro rooting of hardwoods trees with juvenile and mature characteristics, p. 277-296. In K. Niemi & C. Scagel. Adventitious root formation of forest trees and horticultural plants – from genes to applications. Research Signpost, Kerala, India. [ Links ]
Betancourt, A.B. 1999. Silvicultura especial de árboles maderables tropicales. Instituto Cubano del Libro, La Habana, Cuba. [ Links ]
Cerdas, L.V., M. Dufour & V. Villalobos. 1998. In vitro organogenesis in Albizia guachapele, Cedrela odorata and Swietenia macrophylla (Fabaceae, Meliaceae). Rev. Biol. Trop. 46: 225-228. [ Links ]
De Klerk, G.J. 2002. Rooting of microcuttings: theory and practice. In Vitro Cell. Dev. Biol. Plant 38: 415-422. [ Links ]
De Klerk, G.J., B. Arnholdt-Schmitt, R. Lieberi & K.H. Neumann. 1997. Regeneration of roots, shoots and embryos: physiological and molecular aspects. Biol. Plantarum 39: 53-66. [ Links ]
De Klerk, G.J., M. Keppel, J. Ter Brugge & H. Meekes. 1995. Timing of the phases in adventitious root formation in apple microcuttings. J. Exp. Bot 46: 965-972. [ Links ]
De Klerk, G.J., W. Van Der Krieken & J.C. De Jong. 1999. The formation of adventitious roots: new concepts, new possibilities. In Vitro Cell. Dev. Biol. Plant 35: 189-199. [ Links ]
Favre, J.M. & R. Médard. 1969. Ontogénie des racines adventives chez la vigne (Vitis vinifera L.) cultivée in vitro. Rev. Gén. Bot 76: 455-467. [ Links ]
Feder, N. & T.P. O’Brien. 1968. Plant microtechnique: some principles and new methods. Am. J. Bot. 55: 123-147. [ Links ]
Hartmann, H.T., D.E. Kester & F.J. Davies. 1990. Plant propagation: principles and practices. Prentice Hall, New Jersey, USA. [ Links ]
Ishii, K. & E. Maruyama. 1992. Tissue culture of some tropical tree species in Peru, p. 219-223. In K. Oono, T. Hirabayashi, S. Kikuchi, H. Handa & K. Kajiwara. Plant tissue culture and gene manipulation for breeding and formation of phytochemicals. Proc. Germa-Japanese joint meeting on plant tissue culture. NIAR, Japan. [ Links ]
Maruyama, E., K. Ishii, A. Saito & K. Migita. 1989. Micropropagation of cedro (Cedrela odorata L.) by shoottip culture. J. Jap. For. Soc 71: 329-331. [ Links ]
Millán-Orozco, L. 2006. Micropropagación de cuatro especies maderables tropicales de interés para Colombia, mediante técnicas de cultivo in vitro. Ph. D. Thesis, University of Santiago de Compostela, Spain. [ Links ]
Millán-Orozco, L. & A. Ballester. 2006. Estudio de los factores relacionados con la micropropagación del cedro rosado (Cedrela odorata L.). Rev. Real Acad. Gal. Ciencias 25: 75-87. [ Links ]
Murashige, T. & F. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plantarum 15: 473-479. [ Links ]
Naija, S., N. Elloumi, N. Jbir, S. Ammar & C. Kevers. 2008. Anatomical and biochemical changes during adventitious rooting of apple rootstocks MM 106 cultured in vitro. CR Biologies 331: 518-525. [ Links ]
Omar, S., K. Godard, A. Ingham, H. Hussain, V. Wongpanich, J. Pezzuto, T. Durst, C. Eklu, M. Gbeassor, P. Sánchez-Vindas, L. Doveda, B.J.R. Philogene & J.T. Arnason. 2003. Antimalarian activity of gedunin and 7-methoxygedunin and synergistic activity with dillapiol. Ann. App. Biol 143: 135-141. [ Links ]
PAFC, 1997. Plan de Acción Forestal para Colombia. Departamento Nacional de Planeación y Ministerio de Agricultura, Bogotá, Colombia. [ Links ]
Ross, M.K., T.A. Thorpe & J.W. Costerton. 1973. Ultrastructural aspects of shoot initiation in tobacco callus cultures. Am. J. Bot. 60: 788-795. [ Links ]
Syros, T., T. Yupsanis, H. Zafiriadis & A. Economou. 2004. Activity and isoforms of peroxidases, lignin and anatomy, during adventitious rooting in cuttings of Ebenus cretica L. J. Plant Physiol. 161: 69-77. [ Links ]
Thorpe, T.A. 1994. Morphogenesis and regeneration, p. 17-36. In I.K. Vasil & T.A. Thorpe. Plant cell and tissue culture. Kluwer Academic, Dordrecht, The Netherlands. [ Links ]
Thorpe, T.A. 2006. History of plant tissue culture, p. 9-32. In V.M. Loyola-Vargas & F.Vázquez-Flota. Plant cell culture protocols. Humana, Totowa, New Jersey, USA. [ Links ]
Torrey, J.A. 1966. The initiation of organized development in plants, p. 39-91. In M. Abercrombie & J. Brachet. Advances in morphogenesis. Academic, New York, USA. [ Links ]
Zhou, J., H. Wu, & G.F. Collet. 1992. Histological study of initiation and development in vitro of adventitious roots in minicultures of apple rootstocks of M26 and EMLA9. Physiol. Plantarum 84: 433-440. [ Links ]
Correspondencia a: Liliana Millán-Orozco, Elena Corredoira & Maria del Carmen San José. Laboratory of Plant Physiology, Instituto de Investigaciones Agrobiológicas de Galicia, CSIC, Avda. de Vigo s/n, 15705 Santiago de Compostela, Spain; lmilln@yahoo.com, elenac@iiag.csic.es, sanjose@iiag.csic.es
Received 12-III-2010. Corrected 30-VIII-2010. Accepted 29-IX-2010.