Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.57 n.3 San José Sep. 2009
Humoral response of paracoccidioidomycosis sera in hamsters with different Venezuelan isolates
Lilian M. Spencer1, Guaniri Mateu1, Sylvia Magaldi2, Fabiola García3 & Sofia Mata-Essayag2
1. Departamento de Biología Celular, Universidad Simón Bolívar, Caracas, Venezuela. Apartado 89000, Caracas 1080, Venezuela. Tel-fax: +58-212-9064221; lspencer@usb.ve
2. Sección de Micología Médica, Instituto de Medicina Tropical, Universidad Central de Venezuela, Caracas, Venezuela.
3. Cátedra de Micología, Escuela de Bioanálisis, Universidad Central de Venezuela, Caracas, Venezuela.
Abstract: Humoral response of paracoccidioidomycosis sera in hamsters with different Venezuelan isolates. Paracoccidioidomycosis (PCM) is a progressive systemic mycosis caused by the fungus Paraccocidioides brasiliensis (Pb), endemic to Venezuela and Latin America. In this study, eight different Venezuelan isolates obtained from patients with PCM, were inoculated intraperitoneally in Syrian hamsters (Cricetus auratus) and studied by immune-serum. Each strain was collected by gently scraping the surface of the culture medium (Sabouraud Dextrose Agar) and suspended in 3ml of 0.15 M phosphate-buffered saline. The antigen obtained was called Paraccocidioides brasiliensis Crude Antigen (CAP). Immunoblotting results showed that the immune-sera from hamsters recognized at least 3 bands: one over 200 kDa, and two of 80 and 15-20 kDa. This study suggests that IgG anti-CAP can reveal a significant variability in the eight Venezuelan isolates. Sera from 88 infected hamsters were evaluated by ELISA with eight different CAPs and Western blot with CAP 37383. ELISA results showed that, the antigen of the virulent isolate 37383 had the highest percentage (38%) of positivity, while the non-virulent isolate 1458 had the lowest one (13.6%). Furthermore, scanning densitometry revealed that the isolate 37383 had less bands than the non-virulent isolates. These results suggest that the ELISA test with CAP 37383 can detect circulating antibodies, and that this virulent isolate may be useful for the diagnosis of PCM, and to monitor disease responses to treatments. Rev. Biol. Trop. 57 (3): 505-513. Epub 2009 September 30.
Key words: humoral response, Paracoccidioides brasiliensis, paracoccidioidomycosis, Syrian hamsters, Venezuelan isolates, ELISA, Immunoblotting.
Paracoccidioidiomycosis (PCM) has been recognized as a public health problem in Latin America, being the most prevalent human deep mycosis in the region. It is a progressive systemic granulomatous disease caused by the fungus Paraccocidioides brasiliensis (Pb) (Brummer et al. 1993). Diagnosis of PCM includes direct observation of the characteristic multiple-budding cells in clinical material, tissue section of biopsy specimens and/or culture. Serology has become a vital instrument for the diagnosis of PCM infection and the identification of antibody response has proven to be very useful. However, some crude antigens have low sensibility and show cross- reactivity with other mycosis. Using different immunodiagnostic tests, several authors have reported different recognition patterns by the antibodies in immunoblotting assays using low virulent and virulent isolates (Camargo et al. 1991, Casotto et al. 1991, Blotta & Camargo 1993, Vaz et al. 1992). Successful application of a serological test for diagnosis relays on the quality of the antigenic preparation. Since purified proteins may provide more specific results (Vaz et al. 1992).
Syrian hamsters (Cricetus auratus, Waterhouse) are valuable experimental models of human disease by Paraccocidioides brasiliensis (Salfelder et al. 1968, Iabuki & Montenegro 1979, Peracolli et al. 1982, Borelli 1984). The aim of this work is the study of the humoral immune response in these animals using crude soluble antigens (CAP) from 8 different isolates of Venezuelan patients, looking for the best antigen for diagnostic purposes.
Materials and methods
The 88 young adult males Syrian hamsters (weighing approximately 50-80 g) were supplied by the Institute of Tropical Medicine, Universidad Central de Venezuela.
Eight isolates of P. brasiliensis (37383, 231a12, 34667, 2511a, 9860, 231a20, 97203 and 1458) were used, preserved on Sabouraud dextrose agar at room temperature at the Medical Mycology Section, IMT. Also, an isolate of Histoplasma capsulatum (10023) was employed for the cross-reaction experiment. All were isolated from human cases.
The inoculum was prepared using a fragment of each isolate transferred and homogenized in a mortar and mixed using 5 ml of phosphate-buffered saline (PBS). The fungal concentration was determined by counting the number of cells in a Neubauer Chamber and colony forming unit (CFU/ml) in each culture. The concentration was adjusted to 1.4 x 106 CFU of fungal elements per ml of suspension.
Eleven hamsters per group of each isolates were inoculated intraperitoneally with 1ml of homogenized suspension of fungal of P. brasiliensis. Another group was inoculated with H. capsulatum. Ten more healthy hamsters were used as control. Water and food were allowed ad libitum. The animals that did not die before four months from the inoculation were sacrificed under deep anesthesia with ether and exsanguinated by cardiac puncture to collect the serum.
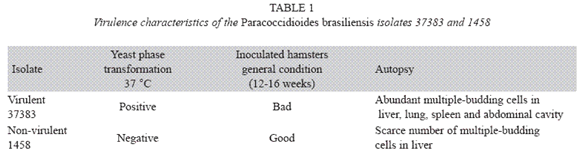
The virulence of the eight isolates was determined on the base of yeast phase transformation (Carbonell & Rodriguez 1965), clinical response of the inoculated animals and by the results of animal necropsy.
Antigen preparation: Each isolate was collected by gently scraping the medium surface and suspended in 3 ml of 0.15 M PBS, centrifuged at 3 000 g per 30 minutes at 4 °C. These antigens were called Paraccocidioides brasiliensis Crude Antigen (CAP) (37383, 231a12, 34667, 2511a, 9860, 231a20, 97203, 1458) and a Histoplasma capsulatum Crude Antigen (CAH) (10023). The antigens were prepared in two conditions: with and without protease inhibitors and their protein concentrations were determined using the Bradford method (Bradford 1976). A cocktail of protease inhibitors containing 1mM PMSF (phenylmethylsulfonyl fluoride, Sigma), 1 mM TLCK (tosyl-lysine-chloromethyl-ketone, Sigma) and 1 mM Leupeptin was added to the suspension of fungi.
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE): CAPs were analyzed by SDS-PAGE on 12.5% w/v polyacrylamide gels (Laemmli 1970). Protein samples were solubilized in SDS sample buffer (0.15M Tris-Cl, pH 6.8, 4.6% SDS, 23% glycerol, and 0.2M DTT in 0.1% w/v bromophenol blue) and heated at 100 °C for 5 min. Proteins samples 4μg track were loaded onto a SDSPAGE gel; the same concentration of protein was loaded for each isolated. The SDS-PAGE gel consisted of a separating gel (12.5% w/v acrylamide/bis-acrylamide, 0.37M Tris-Cl, pH 8.8, 0.1% w/v SDS, set by the addition of 0.3% w/v TEMED, 0.03% w/v ammonium persulphate) with a 3% stacking gel (3% w/v acrylamide/bis-acrylamide, 0.1M Tris-Cl pH 6.8, 0.1% w/v SDS, plus TEMED and 0.05% w/v ammonium persulphate). Electrophoresis was carried out at 20 mA for 1 hour using a Mighty Small II vertical slab gel unit (Bio Rad Instruments). Wide molecular mass markers obtained from BioRad (6.5-200, Kda) were used. After electrophoresis the gel was stained with 0.1 % w/v Coomassie Brilliant Blue R-250 (Sigma) in methanol:water:ethanoic acid (5:5:1) and then destained with a solution of methanol:water:ethanoic acid (1:17:2).
Immunoblotting: After SDS-PAGE, proteins were electrophoretically transferred to nitrocellulose paper (NCP, Schleicher & Schuell, 0.45 µm pore size) to allow immunodetection of the proteins, which could be recognized by the antibodies from sera. The proteins were transferred at 120 mA in a transblotting chamber (Bio-Rad, Instruments), for 1 h at 4°C, using 25 mM Tris-HCl, 150 mM glycine, 20% v/v methanol, by the method of Towbin et al. (1979).
After transfer, the blots were blocked by incubation with a solution of 3% w/v non-fat milk powder in PBS for 5 min at room temperature, and were then washed three times (3x) in PBS, containing 0.05% v/v Tween-20 (Sigma) (PBS/T). Blots were incubated with a solution of primary antibody diluted 1:200 in PBS/T for 1 hour at room temperature. Blots were then washed 3x, and incubated in a solution of affinity purified goat anti-hamster polyvalent immunoglobulin conjugated to peroxidase, at a 1:1000 dilution (Vector Laboratories), for 1 hour at room temperature. Blots were washed again in PBS (3x) and the antibody binding was detected by chemiluminescent substrate Luminol (ECL Detection System, Amershan, UK). Finally, the strips were exposed to Hyperfilm (Amersham, UK) in the dark and developed photographically using the manufactures instructions.
Scanning densitometry: After separation by SDS-PAGE and immunoblotting, each track of the SDS-PAGE and NCP strips were analyzed by scanning densitometry (CS 9000; Shimadzu Corporation Spectrophotometric Instrument, Japan) at 550nm.
Enzyme-linked Immunosorbent Assay (ELISA): ELISA test was used to detect antibodies in the sera of hamsters secreting CAP specific antibodies. Ninety-six well plates (Immulon 4 from Dynatech) were coated with 100µl of antigen solution (CAP) per well, using 10 µg/ml of the antigen solution diluted in coating buffer (0.1 M sodium carbonate/bicarbonate pH 9.6). Plates were incubated overnight at 4°C and washed three times (3x) with PBS containing 0.05% v/v Tween 20 (PBS/T). Plates were then blocked by the addition of PBS containing 1% v/v bovine serum albumin (BSA) for 30 minutes at room temperature, followed by further washing with PBS (3x). One hundred µl of the dilution 1:200 of serum were added to each well and incubated at 37°C for 1 hour. The plates were then washed 3x with PBS and 100µl/well of horseradish peroxidase-conjugated (HRP-) goat biotinylated anti-hamster monovalent immunoglobulin (IgG; H+L) (Vector Laboratories), were added at a 1:500 dilution. After incubation for 1 hour at 37°C the plates were washed again with PBS. Binding of the conjugate to bound antibody was detected by the addition of 100µl of azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS, KPL) as substrate, pH 5.0 containing 0.03%w/v sodium perborate. The reaction was stopped by addition of 50µl 2M H2SO4 per well. The plates were read in Titertek Multiscan MCC/340, using Titer-soft Software (Flow) to measure the Absorbance at 405 nm (Mendes-Giannini et al. 1984). A hamster polyclonal serum against CAP antigens of P. brasiliensis was used as a positive control and 10 normal hamster sera (NHS) were used as a negative control.
Statistical analysis: The statistical analysis was made by Chi square (X2) test, and the significance level used was 0.05.
Results
Among the eight isolates in study the 37383 isolate was the most virulent and the isolate 1458 the least virulent (Table 1). The remaining isolates showed yeast phase transformation at 37°C but the health conditions of the animals were good and on necropsy, a scarce number of multiple-budding cells were found in the lungs and livers.
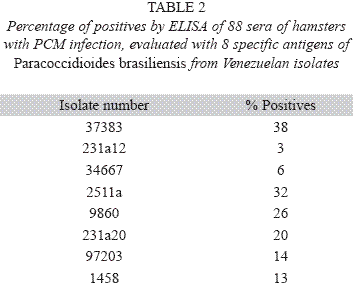
When using the ELISA method to test the eight different isolates as antigens, we found that the isolate 37383 presented the highest percentage of positivity (Table 2). The chi-square value for isolate 37383 was p= 7x10-5, when the null hypothesis was the dependence between the serological test (ELISA) and the clinical diagnosis of PCM of the hamsters and the alternative hypothesis was the independence of clinical observation of PCM. For the study, 88 sera reported by ELISA were evaluated by this method of diagnosis. The sensitivity and specificity of the ELISA test with the antigen 37383 was 60% and 80% respectively. In the same way the p value of the antigen 1458 was p= 9x10-4 and it was related to the clinical observations. In this case the sensitivity and specificity was 28% and 96% respectively. However, the ELISA test with this antigen reported false negatives.
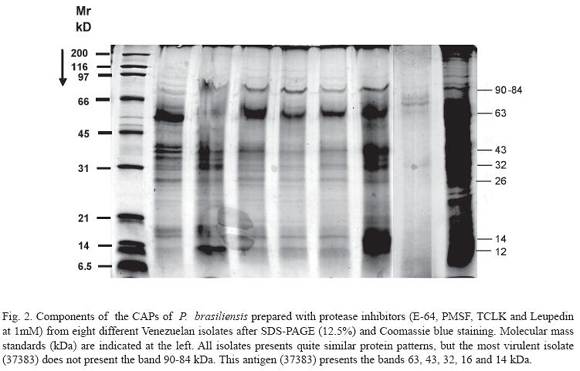
The components of the CAPs of P. brasiliensis from the different Venezuelan isolates were analyzed by SDS-PAGE. At least 9 to 10 bands (120, 110, 105, 97, 73, 60, 40-43, 35, 17 and 12 kDa) were observed after Coomassie blue staining in the majority of the isolates studied (Fig. 1). The components of CAPs prepared with protease inhibitors showed a reduction in the bands to 7 (90-87, 65, 42-40, 35, 28, 15 and 12-13 kDa) (Fig. 2).
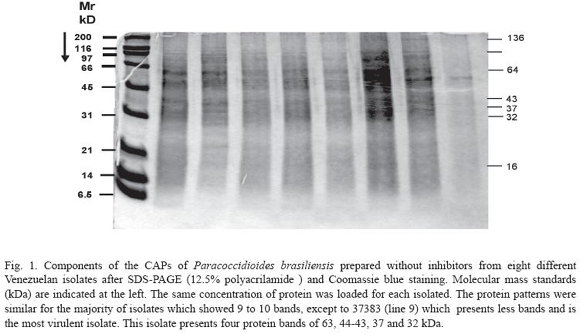
Analyzing the components of the CAPs of the P. brasiliensis prepared without inhibitors from the eight different isolates after SDSPAGE (12.5% polyacrilamide) and Coomassie blue staining, we observed that the protein patterns were similar for the majority of isolates which showed 9 to 10 bands, except isolated 37383 (line 9) which presented fewer bands. This isolate presented four protein bands of 63, 44-43, 37 and 32 kDa (Fig. 1).
Using components of the CAPs of P. brasiliensis prepared with protease inhibitors (E-64, PMSF, TCLK and Leupeptin at 1mM) from the same isolates after SDS-PAGE (12.5%) and Coomassie blue staining, all isolates presented very similar protein patterns, but the most virulent isolate (37383), which did not present a band at 90-84 kDa. This antigen (37383) showed bands at 63, 43, 32, 16 and 14 kDa (Fig. 2).
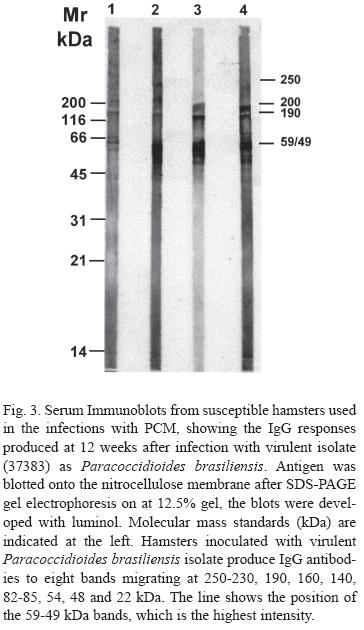
The sera immunoblots from inoculated hamsters, to demonstrate the IgG responses produced after 12 weeks of infection with the virulent isolate (37383), showed that those animals produced IgG antibodies showing eight migrating bands at 250-230, 190, 160, 140, 82-85, 54, 48 and 22 kDa. The highest intensity band was the 59-49 kDa (Fig. 3).
The PCM sera (IgG) recognized eight bands of 250, 190, 160, 140, 85, 54, 48 and 22 kDa with the antigen 37383 prepared with protease inhibitors. The most frequent reactive components for the IgG serum were 190, 160, 85 and 48 kDa. Sera from hamsters with the disease had antibodies reactive with seven antigenic components of P. brasiliensis by immunoblot with the virulent 37383 isolate.
Our results indicate that in an experimental animal model of PCM infection, IgG antibodies were able to recognize similar components of the CAP. Similar results have been previously reported (Vaz 1992).
The literature about the serodiagnosis to PCM has reported crossreactivity between P.brasiliensis and H. capsulatum (Puccia & Travassos 1991). We also evaluated the cross reactivity with an isolated of H. capsulatum (10023) as antigen and obtained 22% of the cross-reaction with sera from hamsters infected with different isolated of P. brasiliensis by the ELISA test (data do not showed).
Discussion
Many serological tests, based on crude extracts obtained from the cultured fungus, have been used in PCM diagnosis (Brummer et al. 1993, Camargo et al. 1989). In these cases, one of the main problems is the high cross reactivity with agents causing other mycosis, especially with histoplasmosis. In addition, the understanding of the uses and limitations of laboratory methods is very important in the correct PCM diagnose (Camargo 2008).
However, this study showed that CAPs preparations from eight different isolates were useful in ELISA test to evaluate the humural responses in hamsters with PCM infection. According to Table 2, the antigen preparations demonstrated different percentages of positives by ELISA test. Moreover, the antigen 37383 with a 38% of positivity, presented a considerable degree of sensitivity (60%) and specificity (80%). Therefore, this high percentage of positivity together with its sensitivity suggests that antigen 37383 is the most appropriate in serological diagnosis. Others studies have reported ELISA results with similar sensitivity and specificity. Mendes-Giannini et al. (1984) reported a 100% of sensitivity and 88% of specificity using yeast filtrate as antigen in ELISA test. Camargo et al. (1984), studying 20 human sera of PCM and 11 of histoplasmosis in an ELISA test, reported similar sensitivity.
In general, immunoblots of serum from hamsters infected with PMC showed differences in the intensities of bands when compared with those obtained from animals infected with a low virulent isolate. The reactions of IgG with most antigen bands were clearly more intense in hamsters infected with the isolate 37383. This result suggests that those bands of 63, 43 and 26 kDa were more antigenic and the humoral response had higher levels. These bands, possibly, correspond to the 70, 43 and 28 kDa bands reported by other authors (Mendes- Giannini et al. 1984, Camargo et al. 1989, Reis et al. 2005, Correa et al. 2006). The protein 43pg is an antigen in conventional and different ELISA tests, recognized by antibodies from heterologous sera and presents reactivity mainly with histoplasmosis sera (Camargo et al. 1994).
In the present study, the antigenic patterns of isolates 231a12, 34667, 2511a, 9860, 231a20, 97203 and 1458 were more complex than those obtained with the isolate 37383 (virulent). We suggest the use of a virulent isolate of P. brasiliensis, such as our isolate 37383, as an antigen for the ELISA test, because this virulent isolate antigen showed the largest percentage of positives samples of infected hamsters and presented one of the highest sensitivities, 60% to 100% of PCM sera with a high specificity (80%).
The production of specific antibodies (IgG) during the course of experimental PCM is related to the severity of the disease. The IgG isotype is significantly elevated in progressive and chronic forms of the disease. We investigated just the IgG anti-P.brasiliensis responses produced in the virulent isolate (37383). In a similar way Vaz et al. (1998), investigated the IgG, IgM, and IgA responses produced in susceptible and persistent mice inoculated with low virulent isolated (Pb 265) and found that the blots with IgG showed a 43-kDa antigen band. Casotto et al. (1991) found antigens of 45 and 48 kDa reactive with antibodies present in patient sera, similar to the 59-49 kDa band, found in the sera of our hamsters. Also, Camargo et al. (1989) using exo-antigen in Western blot for diagnosis of PCM patiens and the IgG response, recognized four bands of 70, 52, 43 and 29 kDa. Our results suggest that the 59-49 kDa component could be the same molecular band as 43-gp kDa reported by other authors.
On the basis of the Chi-square statistical analysis, the ELISA test is a good tool for diagnosis, since it correlates best with the clinical diagnosis.
The results of the analysis by SDS-PAGE could be indicative that a virulent isolate presents fewer components than low virulent isolates. We think that the protease inhibitors are useful in the preparation of the CAPs antigens if they are to be stored for long periods of time, since they are more protected against endogenous proteases than those excreted into liquid culture medium. However, if the antigen is going to be used immediately it is not necessary. Finally, we recommend to first screen with the ELISA test as a diagnostic tool, and confirm in the cases where the ELISA is not clear with Immunoblot test (Camargo et al. 1989).
In general, different authors agree that the cross-reactions are due to the use of unfractionated antigens and they have eliminated the cross-reactivity using the purified molecule of 43gp of P.brasiliensis as an antigen in ELISA tests and reported a rapid, sensitive and specific diagnosis that differentiates between the acute and chronic form the disease (Camargo 2008). This is supported by the works of Albuquerque and colleagues (2005) and Ferreira and coworker (2008); who worked with 43gp antigen treated with sodium metaperiodate to eliminated the carbohydrate epitopes responsible for cross reactions between PCM and histoplasmosis sera.
Actually, tests based in molecular technique present more sensitivity than serologic tests like ELISA. Correa et al. (2006) subcloned the P. brasiliensis p27 gene and used the recombinant protein as antigen in dot blot assay to improve the PCM diagnosis. In this case, they obtained results with a 100 and 98% of sensitivity and specificity respectively (Ortiz et al. 1998). Also, the 43 gp has been used as recombinant protein in PCM diagnosis. Taborda & Camargo (1994), and Cisalpino et al. (1996), found good sensitivity and specificity with the use of recombinant antigens. But in regions where the possibilities doing this kind of assay do not exist, the serologic test is a good alternative to be used in PCM. Establishing the differences in the recognition patterns of antibodies present in any serum with different immunological tests (ELISA, Immunoblot) and a good preparation of antigen allowed us to obtain a useful tool for serological diagnosis of PMC.
Even more, we think that each region should study its own isolates of PCM in order to obtain better diagnosis. Our results suggest that in Venezuela the ELISA test to PCM based on 43 gp molecule could be improved by using a purified antigen of the virulent isolate 37383.
Acknowlegments
We wish to thank Claudia Hartung for technical assistance in culture of Paracoccidioides brasiliensis. Also, we thank Cristina Castelli and Juan Carlos Piña-Crespo for critical reading of this manuscript. This work was supported by Decanato de Investigación de la Universidad Simón Bolívar and Consejo de Desarrollo Científico y Humanístico (CDCH) de la Universidad Central de Venezuela, project N° 09-3454-97.
Resumen
La Paracoccidioidomicosis (PCM), es una micosis sistémica causada por el hongo Paraccocidioides brasiliensis (Pb), endémica en Venezuela y Latino América. En este estudio ocho diferentes aislados venezolanos, obtenidos de pacientes con PCM, fueron inoculados intraperitonealmente en hámsteres y fueron estudiados por ELISA e inmunoblotting. Los antígenos obtenidos de P. brasiliensis fueron llamados, Antígeno Crudo (CAP). Los resultados del immunoblotting mostraron que los sueros inmunes de hámsteres reconocieron al menos tres bandas: una sobre 200, y otras de 80, y 15-20 kDa. Este estudio sugiere que la IgG anti-CAP muestra una variabilidad en los ocho aislados Venezolanos. Sueros de 88 hámsteres infectados fueron evaluados usando ELISA, el antígeno del aislado virulento 37383 mostró el más alto porcentaje de positividad (38%) en los sueros de los hámsteres estudiados. El aislado novirulento 1458 mostró un porcentaje bajo de positividad (13.6%). Además, un escaneo densitométrico reveló que el aislado 37383 tiene menos bandas que el otro aislado no-virulento. Por lo tanto, estos resultados sugieren que el ensayo de ELISA con CAP 37383 puede detectar anticuerpos circulantes y este aislado virulento puede ser útil para el diagnostico de PCM, y para el monitoreo de la respuesta al tratamiento de la enfermedad.
Palabras clave: respuesta humoral, Paracoccidioides brasiliensis, paracoccidioidomicosis, hámsteres Syrian, aislados venezolanos, ELISA, Immunoblotting.
Received 09-IX-2008. Corrected 22-II-2009. Accepted 25-III-2009.
References
Albuquerque C.F., S.H. Marques da Silva & Z.P. Camargo. 2005. Improvement of the specificity of an ELISA-linked immunosorbent assay for diagnosis of Paracoccidioidomycosis. J. Clin. Microbiol. 43: 1944-1946. [ Links ]
Blotta, M.H.S.L. & Z.P. Camargo. 1993. Immunological response to cell-free antigens of Paracoccidioides brasiliensis: Relationship with clinical forms of Paracoccidioidomycosis. J. Clin. Microbiol. 31: 671-676. [ Links ]
Borelli, D. 1984. Micopatología experimental: modelos múltiples. Rev. Fundación José María Vargas 8: 188-92. [ Links ]
Bradford, M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248-254. [ Links ]
Brummer, E., E. Castañeda, A. Restrepo. 1993. Paracoccidioidomycosis: an update. Clin. Microbiol. Rev. 6: 89-117. [ Links ]
Camargo Z.P., J.L. Guesdon, E. Drouhet & L. Improvisi. 1984. Enzyme-linked immunosorbent assay with counterimmunoelectrophoresis and erythro-immunoassay. Mycopathologia. 81: 31-37. [ Links ]
Camargo Z.P., C.S. Unterkircher & L.R. Travassos. 1989. Identification of antigenic polypeptides of Paracoccidioides brasiliensis by immunoblotting. J. Med. Vet. Mycol. 27: 407–412. [ Links ]
Camargo Z.P., C.P. Taborda, E.C. Rodríguez & L.R. Travassos. 1991. The use of cell-free antigen of Paracoccidioides brasiliensis I serological tests. J. Med. Vet. Mycol. 29: 31-38. [ Links ]
Camargo Z.P., J-L. Gesztesi, E.C.O. Saraiva, C.P. Taborda, A.P. Vicente & J.D. Lopes. 1994. Monoclonal antibody capture enzyme immunoassay for detection of Paracoccidioides brasiliensis antibodies in paracoccidioidomycosis. J. Clin. Microbiol. 32: 2377-2381. [ Links ]
Camargo Z.P. 2008. Serology of paracoccidioidomycosis. Mycopathologia 165: 289-302. [ Links ]
Carbonell LM. & J. Rodríguez. 1965. Transformation of mycelial and yeast forms of Paracoccidiodes brasiliensis in cultures and in experimental inoculations. J. Bact. 90: 504-506. [ Links ]
Cassotto M., S. Paris & Z.P. Camargo. 1991. Antigens of diagnostic value in three isolates of Paracoccidioides brasiliensis. J. Med. Vet. Mycol. 29: 243-253. [ Links ]
Cisalpino P., R. Puccia, L. Yamauchi, M. Cano, J.F. da Silveira & L.R. Travassos. 1996. J. Biol. Chem. 271: 45-60. [ Links ]
Correa M.M., A.M. Bedoya, M.P. Guerrero, A. Restrepo & J.G. McEwen. 2006. Diagnosis of paracoccidioidomycosis by a dot blot assay usisng a recombinant Paracoccidioides brasiliensis p27 protein. Mycoses 50: 41-47. [ Links ]
Ferreira, A.P., T. Correa , R. Cunha , M.J. Marquez, M.A. Montesano, M.A. Souza & H.C. Teixeira. 2008. Human serum antibody reactivity towards Paracoccidioides brasiliensis antigens treated with sodium metaperiodate. Rev. Soc. Bras. Med. Trop. 41: 325-329. [ Links ]
Iabuki, K. & M. Montenegro. 1979. Experimental paracoccidioidomycosis in the Syrian hamster: morphology, ultrastructure and correlation of lesions with presence of specific antigens and serum levels of antibodies. Mycopathology 67: 131-141. [ Links ]
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature
227: 680-685.
Mendes-Giannini, M.J.S., M.E Camargo, C.S. Lacaz & A.W. Ferreira. 1984. Immunoenzymatic absorption test for serodiagnosis of paracoccidioidomycosis. J. Clin. Microbiol. 20: 103-108. [ Links ]
Ortiz B.L., S. Diez, M.E. Urán, J.M. Rivas, M. Romero, V. Caicedo, A. Restrepo & J.G. McEwen. 1998. Use of the 27-kilodalton serodiagnosis of paracoccidioidomycosis. Clin. Diagn. Lab. Immunol. 5: 826-830. [ Links ]
Peracolli M.T.S., N.G.S. Mota & M.R. Montenegro. 1982. Morphology and correlation of lesions with humoral and cell-mediated immunity. Mycopathologia. 79: 7-17. [ Links ]
Puccia R. & L.R. Travassos. 1991. 43-kilodalton glycoprotein from Paracoccidioides brasiliensis: immunochemical reactions with sera from patients with paracoccidioidomycosis, histoplasmosis, and Jorge Lobos disease. J. Clin Microbiol. 29: 1610–1615. [ Links ]
Reis B. S., A. Bozzi, F.L.S. Prado, M.C.N. Pereira, F.E. Ferreira, P. Godoy, L. Moro, E.P. Pedroso, M.F. Leite & A.M. Goes. 2005. Membrane and extracellular antigens of Paracoccidioides brasiliensis (Mexo): identification of 28-kDa protein suitable for immunodiagnosis of paracoccidioidomycosis. J. Immunol. Meth. 307: 118-126. [ Links ]
Salfelder K., J. Schuwarz & C. Johnson. 1968. Experimental cutaneous South American blastomycosis in hamsters. Arch. Dermatol. 97: 69-77. [ Links ]
Taborda C. & Z.P. Camargo. 1994. Diagnosis of paracoccidioidomicosis by dot immunobinding assay for antibody detection using the purified and specific antigen gp43. J. Clin Microbiol. 32: 554-556. [ Links ]
Towbin H., T. Staehelin & J. Gordon. 1979. Electrophoresis transfer of proteins from polyacrylamide gels to nitrocellulose sheets: produce and some applications. Proc. Natl. Acad. Sci. Unit. States Am. 76: 4350-4354. [ Links ]
Vaz C.A.C., D.W.R., Mackenzie, V.M. Hearm, Z.P. Camargo, L.M. Singer-Vermes., E. Burge & V.L.G. Calich. 1992. Specific recognition pattern of IgM and IgG antibodies produced in the course of experimental paracoccidioidomycosis. Clin. Exp. Immunol. 88: 119-123. [ Links ]
Vaz C.A.C., L.M. Singer-Vermes & V.L.G. Calich. 1998. Comparative studies on the antibody repertorie produced by susceptible and resistant mice to virulent and nonvirulent of Paracoccidioides brasiliensis isolates. Am. J. Trop. Med. Hyg. 59: 971-977. [ Links ]














