Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.54 suppl.3 San José Dec. 2006
The white band disease type II pathogen in Puerto Rico
D. L. Gil-Agudelo1 *, G.W. Smith2 & E. Weil3
1 Instituto de Investigaciones Marinas y Costeras INVEMAR, Cerro Punta de Betín, P.O. Box 1016, Santa Marta, Magdalena, Colombia. Tel: +57(5) 421 1380 x 141, Fax: +57(5) 431 2986; diego.gil@invemar.org.co
2 University of South Carolina Aiken, Dept. of Biology and Geology. 471 University Parkway, Aiken, SC 29801 USA. Tel: +1(803) 641 3427, Fax: +1(803) 641 3251; smithres@usca.sc.edu
3 University of Puerto Rico, Department of Marine Sciences, P.O. Box 908, Lajas, PR 00667, Puerto Rico. Tel: +1(787) 899 2048 x 241, Fax: +1-(787) 899 5500 / 2630; eweil@caribe.net
* Correspondence author
Received 02-VI-2006. Corrected 02-X-2006. Accepted 13-X-2006.
Abstract: The white band disease type I (WBD-I) epizootic event of the early 1980s resulted in significant changes in the structure and composition of coral communities throughout the wider Caribbean. The disease decimated populations of acroporid corals throughout their geographic distribution and it is still affecting the surviving and recovering populations of these corals in a number of localities in the wider Caribbean. The putative pathogen for this syndrome (WBD-I) was never identified. A second pattern of white band was described later as white band type II (WBD-II). A potential pathogen named Vibrio charchariae was identified but Kochs postulates were never fulfilled. In this work, we present results of a preliminary approach to confirm the identity of the pathogen of WBD-II. During the fall months of 2004, samples of Acropora cervicornis with signs of WBD-II were collected from a small population in Mario reef, an isolated patch reef off La Parguera, southwest coast of Puerto Rico. Bacteria extracted from these samples were isolated in TCBS agar, grown in Glycerol Seawater agar, and then used to inoculate separated, healthy-looking colonies of the same population in the same reef. Isolation, culture, and inoculations of bacteria were conducted under controlled conditions within hours of collection, and no microorganisms that were not already in the reef community were introduced with these experiments. Some of the newly inoculated colonies developed the disease signs within 24 hr. These were subsequently sampled and bacterial re-isolated to be identified, thus complying with the first steps to fulfill Koch s postulates for this disease. Rates of advance of the disease signs varied between 0.5 and 2 cm/day. Preliminary analyses indicated that the potential cause of WBD-II is a Vibrio species very close to Vibrio harveyi, a synonymy of V. charchariae. All inoculated coral colonies that developed the signs of WBD-II, behaved as the naturally infected colonies, and all of them showed no signs of the disease after two months of the inoculation when water temperatures dropped due to winter in the area. Rev. Biol. Trop. 54 (Suppl. 3): 59-67. Epub 2007 Jan. 15.
Key words: White Band Disease Type II, Acropora cervicornis, Vibrio harveyi, Vibrio charchariae, Kochs postulates, field Inoculations.
Outbreaks of white band disease type I (WBD-I) during the 1970s and 1980s (Gladfelter 1982) represent the most devastating epizootic affecting scleractinian corals to date. This disease was responsible for the mortality of more than 90% of the acroporid corals (Acropora palmata and Acropora cervicornis) Caribbean wide (Aronson and Precht 2001). A series of significant ecological events followed, including changes in the general zonation patterns of shallow reefs in the Caribbean (Goreau 1959, Geister 1977), loss of biodiversity due to loss of habitat when the tridimensional spatial structure produced by these species collapsed, and a shift from coral dominated to algal-dominated communities resulting from the combination of this event and the loss of the black sea urchin Diadema antillarum (Hughes 1994, Weil 2004). More than 20 years later, most populations of these acroporids have not recovered and the putative pathogen associated with this epizootic was never fully identified.
A different pattern or phase of this disease in A. cervicornis was described in the late 1990s and was named WBD type II (Ritchie and Smith 1998). It differed from WBD-I by a bleaching band leading the necrotic edge of living tissue. These signs have only been observed in A. cervicornis, and it was not clear if these two patterns were caused by different pathogens, if the disease is expressed differently in different corals, or if the two etiologies represent different phases of the same syndrome (Bythell et al. 2004).
Although several authors have tried to identify the causal agent of both WBD-I and WBD-II, the pathogen(s) have remained elusive for almost 30 years. Using histological techniques, Gram-negative bacterial aggregations were observed in association with A. palmata experiencing signs of WBD-I (Peters et al. 1983). Later, the Gram-negative bacteria Vibrio carchariae was suggested as the putative pathogen responsible of WBD-II (Ritchie and Smith 1995, 1998), although re-inoculation experiments were not performed due to the difficulty of maintaining healthy corals in aquaria, thus not fulfilling Kochs postulates.
Preliminary results from a pilot study conducted in December 2003, in the same reef where the present study was performed, showed the development of signs of WBD-II by one bacterial strain. This strain was sequenced and the results indicate a close relationship (99%) to Vibrio harveyi (after sequencing more than 1000 bp).
The major goals of this project were to isolate potential bacteria from disease colonies, culture them and re-inoculate healthy branches to try to reproduce the signs of WBD-II as a first step to fulfill Kochs postulates for this important coral disease.
Materials and methods
Five branches showing signs of WBD-II were collected using aseptic techniques from a small population of of A. cervicornis in the back, narrow lagoon of Mario Reef, an isolated patch reef off La Parguera, southwest coast of Puerto Rico (66º 59- 67º 06 W and 18º 01- 18º 07 N) in November 2004. Sterile Whirl-pak plastic bags were placed on top of the branch which was then cut. The bag was sealed and placed in a cooler filled with seawater to keep dark conditions and stable water temperatures. Samples were immediately transported to the laboratory at the Magueyes Marine Biology Station of the Universidad de Puerto Rico, Mayaguez, where they were processed within three hours of collection.
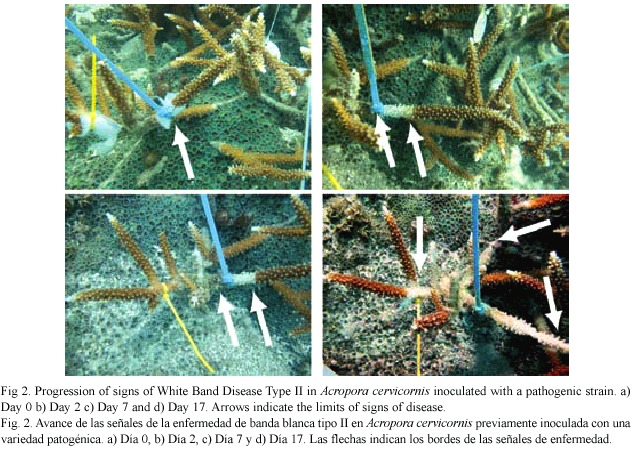
In the laboratory, each of the branches was crushed using a previously flamed sterilized mortar and pestle and 2 ml of filter sterilized (0.22 m) seawater. The resulting mix was homogenized and plated in different dilutions (10-1 to 10-3) on TCBS agar (Difco) and incubated for no more than 24 hr. After incubation, a total of 61 strains were restreaked on Glycerol Seawater Agar (GSW) (modified from Smith and Hayasaka 1982). Fifty six (56) isolated bacterial strains survived this transfer and were inoculated onto healthy A. cervicornis corals by spreading them on pieces (3x3 cm) of sterile gauze. Then, the pieces of gauze were transported to the field in sealed sterile labeled Whirl-pak plastic bags. Inoculations were performed no later than 72 hr after the initial isolation of the strains to avoid the attenuation of pathogenicity of the bacterial strains. Fifty six branches were haphazardly selected in the same population of A. cervicornis from which the disease samples were collected in Mario Reef, and inoculated by placing the small piece of gauze with the inoculum in contact with the live, healthy looking tissue and secured with plastic cable ties (Fig. 1). During these experiments, inoculum concentrations were not controlled. Inoculated coral branches were marked and photographed. After 24 hr, the gauze was removed to avoid further damage to the live tissue, and colonies were photographed and monitored daily for one week and then at different intervals for two weeks by photographing the development of signs and measuring the advance of the disease. Thirty five colonies did not show any signs of stress or disease could be considered controls for the experiment.

For a first screen, each one of the 56 bacterial strains transferred to GSW were inoculated onto only one branch of healthy A. cervicornis. No replications were performed at this stage due to the high number of corals needed and only the presence/absence of disease development was evaluated. After only four days, 20 out of the 55 A. cervicornis colonies inoculated showed distinguishable signs of the development of WBD-II. Eight of these inoculums (the ones showing the faster and most clear disease development) were inoculated by triplicate in haphazardly selected A. cervicornis branches using the same inoculation protocol. Each replicate was placed in colonies several meters apart to avoid the possible effect of genotypic resistance, although, different branches of the same coral colony were used to test different inoculums. The development of the disease signs was photographed and the advance of the dead edge measured at different intervals for over a week (day 4 and 10). Colonies were monitored for the spread of the disease for over two months (day 14, 18, and 37, plus weekly visits where no measurements were performed) until all disease signs arrested during winter. The 35 coral branches inoculated with bacteria that did not show stress or disease signs were in good conditions by the end of the observations. WBD-II signs present in many of the branches of this population were present throughout the experiment and similarly, the signs arrested and disappeared by February 2005.
Four out of the 24 inoculated branches with signs of the disease were collected, crushed, and their bacteria cultivated in TCBS agar as described above. Bacterial colonies with different morphologies were isolated and preserved at -80ºC for future analysis using 50% glycerol and 50% GSW broth. Preliminary sequencing of the 16S rDNA of some of the strains showing the development of signs similar to WBD-II was performed using universal primers (27F and 1392R).
Results
Results of this second study represent the first time signs of WBD-II are consistently reproduced, an important step forward in the determination of the pathogenic agent producing this disease. A total of 61 bacterial strains were observed in TCBS agar and 56 of them were successfully transferred and isolated in GSW agar and inoculated into healthy corals. After two days, 20 inoculated corals showed signs similar to WBD-II, 27 after five days, and 10 of them persisted for more than 18 days (Table 1, Fig. 2). Replication of the inoculation experiments showed that all of the eight bacterial strains selected produced signs of the disease in at least two of the replicates, but the speed of spread of the disease (measured by the distance from the inoculation point to the limit of the healthy and necrotic tissue) was variable in most of them, changing from 0.2 to 1.2 cm/day (Table 2, Fig. 3).
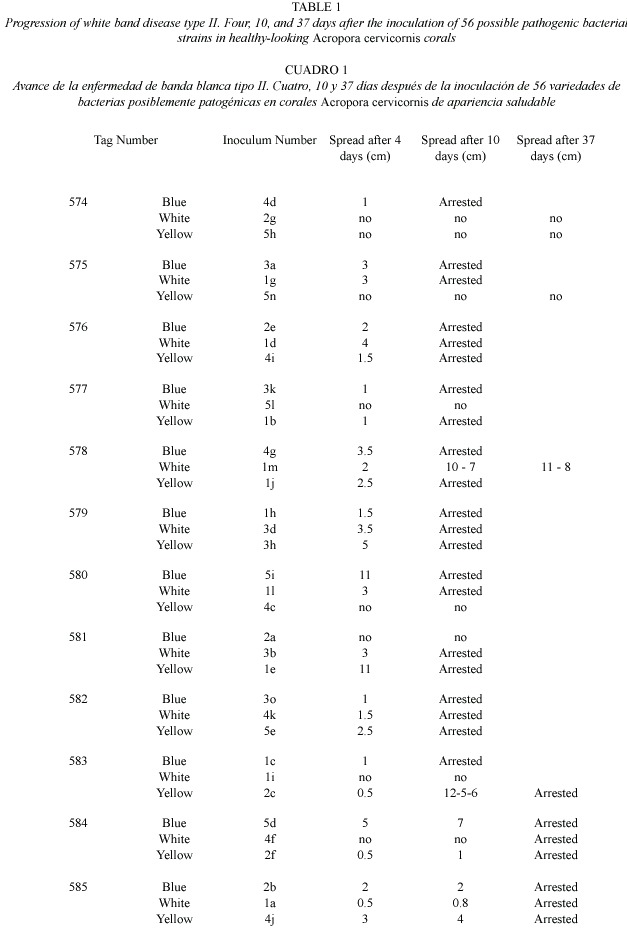
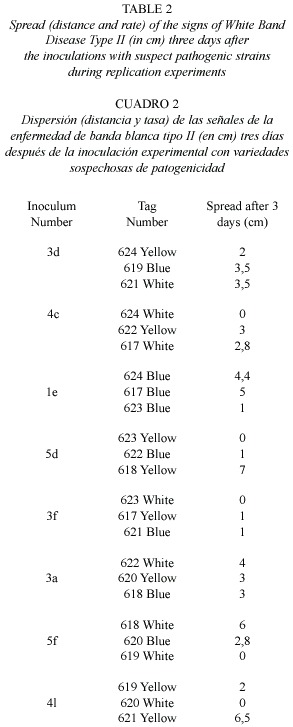

At this point of the experiment, only three of the four Koch´s postulates have been satisfied.
1. The microorganism is present in diseased organisms and absent in healthy ones (postulate partially fulfilled, it has to be corroborated once the identity of the suspected pathogen is established).
2. Suspected microorganism must be isolated and grown in pure culture.
3. The same disease must result after inoculation in healthy hosts.
The fourth postulate (re-isolation of the microorganism) is not presented here because the identification of bacteria inoculated and re-isolated are still under way. Further work is being performed in order to identify the re-isolated bacteria from infected corals using sequencing of the 16S rDNA and to relate them with the inoculated strains.
Results from pilot studies in December 2003, indicated that one bacterial strain inoculated in one isolated branch of A. cervicornis developed the characteristic signs of WBD-II and that after sequencing more than 1000 bp, this strain was very close (99%) to Vibrio harveyi.
Discussion
Although identification of the bacterial strains responsible for producing signs of WBD-II are still under way, preliminary evidence points to the involvement of V. harveyi the infectious agent, supporting results from 2003 pilot experiments. These results also agree with those reported by Ritchie and Smith (1998), which first established V. carchariae (a synonymous of V. harveyi) as the most probable pathogen of this disease. This is, however, the first time that signs of WBD-II have been successfully reproduced in several replicates under laboratory and/or field conditions.
This is not the first time that a Vibrio species has been related to coral diseases. Vibrio shilonii (=V.shiloi ) for instance, has been held responsible of bacterial bleaching in the Mediterranean (Kushmaro et al. 1997), and V. coralliilyticus infects and kills Pocillopora damicornis in the Indo-Pacific (Ben-Haim et al. 2003).There is some evidence that yellow blotch syndrome in the Caribbean is also caused by a Vibrio (Cervino et al. 2004) Vibrio is therefore, one of the most important coral pathogens.
Rates of spread of the disease obtained during this experiment are comparable with the ones observed under natural conditions, where spread was estimated between less than 1 cm/day to a maximum of 2 cm/day for WBD-I (Gladfelter 1982) and to a maximum of 5 cm/day for WBD-II (Ritchie and Smith 1998). Differences observed in the rate of spread of the disease throughout the coral branches might be the result of genetic differences als, bacterial strains, or the combination of both of them. During these experiments, inoculum concentrations were not controlled. These concentrations might have also influenced the rate of spread of the disease and also increased the probability of acquiring the disease by the coral. For future experimentation this factor / variable should be controled to assess if centration is related to rate of disease infection and/or rates of advance of the disease over the coral surface. Concentration of pathogen may also affect the susceptibility of the coral tissue to particular pathogens (defense mechanism may be overwhelmed by high concentrations of pathogens for example).
Regarding field inoculations: Field inoculations with putative pathogenic microorganisms have positive elements for the study of coral diseases. For example, environmental conditions similar to conditions required for disease progression are likely to occur in the field if the disease is present. Other advantages include the availability of high number of corals for trials, the possibility of performing several trials in corals that are genetically different and the possibility of observing the full development of the disease under natural conditions, which is sometimes difficult to observe in small laboratory trials. It must be acknowledged that field inoculations have risks such as the release of large amounts of putative pathogenic bacteria into the system. Because of this, special considerations have to be taken when planning and performing these experiments. First of all, bacterial strains should only be inoculated on the same reefs where they were originally extracted. In this way, the introduction of bacterial species into new areas will be avoided. Also, inoculation of bacteria should be done only in areas with high incidence of the disease that is being studied and the matrix for the inoculation (in this case the piece of gauze) must be collected and disposed properly. This reduces the possibility of spread of the possible pathogens to areas with low incidence. The number of experiments should be carefully planned to avoid excessive inoculation of organisms that might create an unexpected outbreak of the disease being studied. It is also important to continue monitoring the inoculated corals as well as the area where the inoculations were performed to detect possible outbreaks of the disease. In our case, an additional factor, water temperature drop, was taken into consideration to prevent dispersion and potential outbreaks. Field observation have shown that several diseases, including WBD-II arrest and disappear from colonies during winter (Weil 2004, Gil-Agudelo et al. 2004), when water temperatures are the lowest.
All the steps described above were followed during the present project to minimize the risk of spread of this disease to other areas or to create outbreaks of the disease.
Acknowledgments
This work was supported by the Khaled Bin Sultan Living Oceans Foundation, the National Oceanic and Atmospheric Administration Coastal Ocean program (NOAA-CRES-0648- 0384 and NA17OP2919), the National Science Foundation (OCE-0326269), and the Coral Disease Working Group of the World Bank-GEF Coral Reef Targeted Research and Capacity Building project. Logistical support and laboratory space was provided by the Department of Marine Sciences, University of Puerto Rico and the Department of Biology and Geology of the University of South Carolina at Aiken. We acknowledge Kim Ritchie for sequencing of an early putative pathogenic strain. This is a contribution No. 975 of INVEMAR.
Resumen
El evento epizoótico de la enfermedad de banda blanca tipo I (WBD-I) al principio del decenio de 1980 causó cambios significativos en la composición de las comunidades coralinas a todo lo largo del Gran Caribe. La enfermedad eliminó altas proporciones de las poblaciones de acropóridos y aún hoy continua afectando la recuperación de sus poblaciones en muchas localidades. El agente causante de esta enfermedad (WBD-I) nunca fue identificado. Un conjunto de características diferentes de esta enfermedad fue descrito en 1998, como banda blanca tipo II (WBD-II), y la bacteria Vibrio charchariae fue identificada como la posible causante. Sin embargo, los postulados de Koch nunca se cumplieron. En este trabajo presentamos los resultados de estudios preliminares para identificar el agente causante de WBD-II en el Caribe. Durante los meses de otoño del 2003 y 2004, recolectamos muestras de Acropora cervicornis con signos de WBD-II en Mario, un arrecife de parche aislado en La Parguera, costa sur occidental de Puerto Rico. Las bacterias extraídas de estas muestras fueron aisladas en agar TCBS, criadas en agar Glicerol Agua de mar y luego utilizadas para infectar colonias separadas sin signos de enfermedad en la misma población y localidad. El aislamiento, cultivo e inoculación de bacterias se hizo en condiciones controladas, y no introdujimos ningún microorganismo que no hubiera estado previamente en el arrecife. Algunas de las colonias inoculadas desarrollaron signos de la enfermedad en 24 hr. Tomamos muestras de estas colonias y las bacterias fueron nuevamente aisladas para ser identificadas y así completar los postulados de Koch. Las tasas de avance de los signos de la enfermedad variaron entre 0.5 y 2 cm/día. Preliminarmente confirmamos que la causa de WBD-II es una especie de Vibrio muy cercana a Vibrio harveyi, sinónimo de V. charchariae. Todas las colonias coralinas inoculadas que desarrollaron signos de WBD-II se comportaron como las colonias infectadas naturalmente y ninguna de ellas presentó signos de la enfermedad al cabo de dos meses, cuando las temperaturas del agua descendieron con el invierno.
Palabras clave: Enfermedad de Banda Blanca Tipo II, Acropora cervicornis, Vibrio harveyi, Vibrio charchariae, postulados de Koch, inoculación en el campo.
References
Aronson, R. B. & W. F Precht. 2001. White-band disease and the changing face of Caribbean coral reefs. Hydrobiol. 460: 25-38. [ Links ]
Ben-Haim Y, F. L. Thompson, C. C. Thompson, M. C. Cnockaert, B. Hoste, J. Swings & E. Rosenberg. 2003. Vibrio coralliilyticus sp. nov., a temperature-dependent pathogen of the coral Pocillopora damicornis. Int. J. Syst. Evol. Microbiol. 53: 309-315. [ Links ]
Bythell, J., O. Pantos & L. L. Richardson. 2004. White plague, white band, and other "white" diseases, p. 351-365. In I. Rosenberg & Y. Loya (eds.). Coral Health and Disease. Springer, Berlin. [ Links ]
Cervino, J. M., R. L. Hayes, S. W. Polson, S. C. Polson, T. J. Goreau, R. J. Martínez & G. W. Smith. 2004. Relationship of Vibrio species infection and elevated temperatures to yellow blotch/band disease in Caribbean corals. Appl. Environ. Microbiol. 70: 6855-6864. [ Links ]
Geister, J. 1977. The influence of wave exposure on the ecological zonation of Caribbean reef corals. Proc. 3rd Int. Coral Reef Symp., Miami 1: 23-29. [ Links ]
Gladfelter, W. B. 1982. White-Band disease in Acropora palmata: implications for the structure and growth of shallow reefs. Bull. Mar. Sci. 32: 639-643. [ Links ]
Goreau, T. F 1959. The ecology of Jamaican coral reefs. I. Species composition and zonation. Ecology 40: 67-90. [ Links ]
Hughes, T. P 1994. Catastrophes phase shifts and large-scale degradation of a Caribbean coral reef. Science 265: 1547-1551. [ Links ]
Kushmaro A., E. Rosenberg, M. Fine & Y. Loya. 1997. Bleaching of the coral Oculina patagonica by Vibrio AK -1. Mar. Ecol. Prog. Ser. 147: 159-165. [ Links ]
Peters, E. C., P. P Yevich & J. J. Oprandy. 1983. Possible causal agent of white band disease in Caribbean acroporid corals. J. Invertebr. Pathol. 41: 394-396. [ Links ]
Ritchie, K. B. & G. W. Smith. 1995. Preferential carbon utilization by surface bacterial communities from water mass, normal, and white-band diseased Acropora cervicornis. Mol. Mar. Biol. Biotechnol. 4: 345-352. [ Links ]
Ritchie, K. B. & G. W. Smith. 1998. Type II white-band disease. Rev. Biol. Trop. 46 (Suppl. 5): 199-203. [ Links ]
Smith, G. W. & S. S. Hayasaka. 1982. Nitrogenase activity associated with Holodule wrightii roots. Appl. Environ. Microbiol. 143: 1244-1248. [ Links ]
Weil, E. 2004. Coral reef diseases in the Wider Caribbean, p. 35-668. In E. Rosemberg & Y. Loya (eds.). Coral Reef Health and Diseases. Springer-Verlag Berlin.














