Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.51 n.3-4 San José Sep. 2003
Ceratitis capitata (Diptera: Tephritidae)
R.D. Briceño
Escuela de Biologia, Universidad de Costa Rica, Ciudad Universitaria, Costa Rica; rbriceno@biologia.ucr.ac.cr; Tel: (506)2074367; Fax: (506)2074216.
Received 07-IV-2003. Corrected 15-VIII-2003. Accepted 01-IX-2003.
Abstract
Males of the mutant strains (blind, vestigal-winged) of the Mediterranean fruit fly (medfly), Ceratits capitata (Wiedmann) showed differences in behavior compared with control (mass-reared) males. Mutant males made fewer mating attempts and achieved fewer matings than control males. Vestigal-winged females copulated less frequently with both mutants. Blind males climbed rather than jumped onto females and copulated in very low numbers compared with control and vestigal males. Blind females copulated normally with control, males and in very low numbers with both types of mutant males.
Key words: Courtship behavior, medfly, Ceratitis capitata, mutants.
Males of the Mediterranean fruit fly, Ceratitis capitata, produce an array of stimuli that the female can detect before accepting or rejecting the male. Courtship begins with the release of pheromones and continuous wing vibration, followed by intermittent wing buzzing (possibly combined with the release of pheromones) and head rocking that causes the males aristae to tap those of the female (Briceño and Eberhard 2000); these can be display several dimorphic characters of the male, which include eye and face colors, and the capitate bristles (Feron 1962, Webb et al. 1983, Briceño et al. 1996, Mendez et al. 1998).
The stimuli involved in the courtship behavior of Ceratitis capitata that induce acceptance by the female are only poorly understood (Eberhard 1999). There is only fragmentary experimental evidence addressing intraspecific visual, acoustic and chemical communication. When male wings were removed, the percentage of females inseminated after three days dropped by about half (Keiser et al. 1973). Mating was inhibited almost totally when both antennae were removed from female medflies (Nakagawa et al. 1973) but this could alter visual, auditory and chemical stimulation of the female. Removal of female aristae resulted in less frequent mounting and copulation (Miranda 2000). The aristae are associated with movement and sound reception (Wigglesworth 1965, Bennet-Clark 1971, Ewing 1989) and probably also tactile stimuli (Briceño and Eberhard 2002). Visual communication was modified by genetic alteration of male eye color (with the apricot gene) which resulted in reduced male mating success (Rössler 1980). Elimination of all visual stimuli by keeping flies in darkness reduced insemination rates by a factor of more than 10 (Keiser et al. 1973) but the possible roles of males and females in this
reduction were not checked. When the capitate anterior orbital bristles of males flies were removed, presumably modifying the visual stimuli received by the female, the female was more likely to attack males and avoid mounting (Mendez et al. 1998)
The study of mutations that affect phenotypic traits can be useful in understanding animal behavior (Hall 1994), and studies using mutant flies in Tephritidae are lacking to date. The study of flies with point mutations that affect either emission or reception of signals promises to improve our understanding of cues which trigger different behavior patterns during courtship. This paper reports on the effects on sexual behavior of vestigal wings and blind mutations in C. capitata.
Materials and methods
The flies were from strains kept in the rearing facilities of the International Atomic Energy Agency in Seibersdorf, Austria. The collection and rearing of mutants from natural populations was begun more than 10 years ago (Röessler 1992).
The mutant strains used were derived from two recessive mutants in field material in Israel, and initially reared in the Cohen Institute for Biological Control (CMBI): white eye (we) located on chromosome 5 found in 1989, and vestigial wings (vg-wp) located on chromosome 4 found in 1990. The control flies were from the Egypt II strain (egII).
The adults were separated by sex 24 hours after emergence, and kept for 4-6 days prior to initiating the experiments in small plastic boxes (16 cm long x 19.5 high x 10.5 wide containing about 100 flies fed with hydrolyzed protein. In the first series of experiments, a pair of virgin flies was placed in a petri dish (13.7 cm diam., 1.8 cm deep). The petri dishes were placed on a glass plate that could be rotated, and the flies were observed from below under artificial illumination, at 24 ºC (10 pairs were observed simultaneously). The male of each pair was placed in the dish 5 min before the female. Observations were initiated at 9 AM and ended at 3 PM. A small microphone (Senheiser system MZK 802V) was inserted through a hole in the ceiling of the chamber to detect sound production.
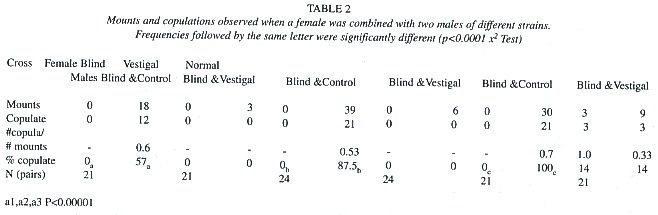
Courtship, mounting and copulation behavior of all flies was noted. In the second series of experiments each petri dish contained one female and two males of different strains. Chi squared tests were used for statistical analyses.
Results
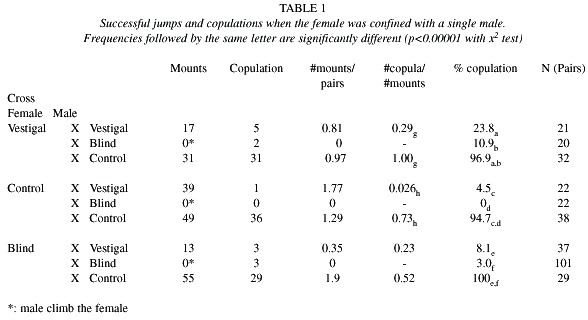
Males of all strains raised the tip of the abdomen with the everted rectal epithelium in the pheromonal calling position (Feron 1962) and all except blind males turned toward females when they passed nearby. Vestigial-winged males did not, however, produce the sustained wing vibration produced by the other strains, although they did lower their abdomen and direct the tip anteriorly like the others. Vestigial-winged males also moved their relatively small wings forward and backward, as did control flies during the succeeding stage of normal courtship; there was intermittent wing buzzing but no sound accompanying these movements was detected by the microphone. Blind individuals of both sexes did not show any response to other flies moving nearby and males did not "track" the female, as was common in the other strains. Blind males did perform continual wing vibration and intermittent wing buzzing, suggesting that these behaviors do not always need visual stimuli to be released. Head rocking behavior, which normally accompanies buzzing, was absent. Blind males never attempted to jump onto females
(Table 1), but they did occasionally climb onto females. Blind females copulated in normal numbers with control males.
The two series of experiments (Table 1, 2) produced similar results, though significance levels were lower in the 2 nd series, presumably due to small sample sizes. Both control and vestigal males mounted all three types of females more often than blind males, and vestigal males mounted fewer females than normal males (Table 1). Control males were more likely to mate after mounting than vestigial males.
Discussion
The visual signals emitted by females (direction of movement, distance and angle with respect to a male) appear to activate and direct male courtship behavior (Briceño et al. 1996, Briceño and Eberhard 2000). The sharply reduced mounting and copulation rate of blind males may thus be due to lack of visual stimuli from the female. Although blind males sometimes courted, lack of proper orientation and timing, may reduce rates of female approach and acceptance. In Drosophila the white eye mutant and optomotor blind males (omb) court in a mediocre manner, largely the result of their poor visual acuity (Stuverant 1915, Geer and Green 1961, cited by Hall 1994). As in Drosophila, male responses are probably important in medfly courtship because they promote female orientation and attraction to the nearly immobile male. The effect of lack of visual stimuli in the female is quite different. Blind females copulated at the same rates with control males as did other females. Presumably other stimuli from those males (possibly chemical, auditory, tactile and air flow) compensated for the lack of visual stimuli perceived by these female.
Female preference for control males over vestigial males could have been due to any one of several differences in stimuli including visual, auditory and chemical (mutant males would be less able to waft odor toward the female).
The vestigial-wing mutation is thought to also reduce or abolish development of the thoracic muscles responsible for raising the wing (Rössler and Rosenthal 1988), and this may be the reason why the males of this strain did not perform sustained wing vibration, and why they did not produce sound.
Acknowledgements
Thanks to J. Hendrichs for inviting me to work with medflies mutants, A. Robinson for allowing me access to IAEA laboratory facilities in Seibersdorf, Austria, to J. Lobo for help with statistical analysis, and William Eberhard and J. Monge for suggestions on previous drafts. The International Atomic Energy Agency and "Vicerrectoria de Investigación" of the University of Costa Rica provided financial support.
Resumen
Machos mutantes (ciegos, alas vestigiales) de la mosca del mediterráneo Ceratitis capitata (Wiedmann) mostraron diferencias en conducta comparados con los machos testigo (cría masiva). Los machos mutantes, realizaron menos intentos por aparearse y lograron menos apareamientos que los machos testigo. Las hembras con alas vestigiales, copularon menos con ambas clases de mutantes. Los machos ciegos, subieron en lugar de saltar sobre las hembras y copularon en números muy bajos comparados con los machos testigo y con los de alas vestigiales. Las hembras ciegas, copularon de forma normal con los machos testigo y en números muy bajos con ambos tipos de machos mutantes.
References
Bennett-Clark, H.C. 1971. Acoustics of insect song. Nature 234: 255-259. [ Links ]
Briceño, R.D. & W. Eberhard. 2002 Decision during courtship by male and female medflies, Ceratitis capitata (Diptera: Tephritidae); correlated changes in male behavior and female acceptance criteria in mass-reared flies. Florida Entomol. 85: 14-31. [ Links ]
Briceño, R.D. & W. Eberhard. 1998. Medfly courtship duration: a sexually selected reaction norm changed by crowding. Ethol. Ecol. & Evolut. 10: 369-382. [ Links ]
Eberhard, W. 1999. Sexual behavior and sexual selection in the mediterranean fruit fly Ceratitis capitata (Dacinae: Ceratidini). In M. Aluja & A.L. Norrbom (eds.). Fruit Flies (Tephritidae) Phylogeny and Evolution of Behavior. CRC. pp. 457-487. [ Links ]
Briceño, R.D. & W. Eberhard. 2002. Courtship in the med-fly, Ceratitis capitata, includes tactile stimulation with the male´s aristae. Entomol. Exp. Appl. 102: 221-228. [ Links ]
Ewing, A.W. 1989. Arthropod bioacustics: neurobiology and behavior. Cornell University. Ithaca, New York. 260 p. [ Links ]
Feron, M. 1962. L´instinct de reproduction chez la mouche mediterraneenne des fruits Ceratitis capitata Wied (Dipt. Tephritidae). Comportemente sexuel. Comportement de ponte. Rev. Pat. Veg. Entomol. 41: 1-129. [ Links ]
Hall, J.C. 1994. The mating of a fly. Science 64: 1702- 1714. [ Links ]
Keiser, I., R. Kobayashi, D. Chambers & E. Schneider. 1973. Relation of sexual dimorphism in the wings, potential stridulation and illumination to mating of oriental fruit fly, melon flies, and Mediterranean fruit fly in Hawaii. Ann. Entomol. Soc. Am. 66: 937-941. [ Links ]
Mendez, V., R.D. Briceño & W. Eberhard. 1998. Functional significance of the capitate supra-fronto-orbital bristles of male medflies (Ceratitis capitata)( Diptera, Tephritidae). J. Kansas Entomol. Soc. 71(2): 164-174. [ Links ]
Miranda, X. 2000. Sexual dimorphism in the arista of Ceratitis capitata (Diptera, Tephritidae) and its possible importance in courtship. NY Entomol. Soc. 108: 339-348. [ Links ]
Nakagawa, S., G.J. Farias, D. Suda & D.L. Chambers. 1973. Mating Behavior of the mediterranean fruit fly following excision of the antennae. J. Economic Entomol. 66: 583-584. [ Links ]
Rössler, Y. & H. Rosenthal. 1988. Genetics of the Mediterranean fruit fly (Diptera: Tephritidae): Eye color, eye shape and wing mutations. Ann. Entomol. Soc. Amer 81: 350-355. [ Links ]
Rössler, Y. & H. Rosenthal. 1992. Genetics of the Mediterranean fruit fly, (Diptera: Tephritidae): morphological mutants on chromosome five. Ann. Entomol. Soc. Amer. 85: 525-531. [ Links ]
Rössler, Y. 1980. Sexual competitiveness of males of the Mediterranean fruit fly, Ceratits capitata (W.)(Diptera: Tephritidae), carrying a Y-chromosome translocacion. Bull. Entomol. Res. 70: 649-656. [ Links ]
Wigglesworth, V.B. 1965. Principles of Insect Physiology. Methven, London. 741 p. [ Links ]