Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.51 n.3-4 San José Sep. 2003
Atlantic forest Rubiaceae: ecology and phylogenetic constraints
Ivonne SanMartin-Gajardo & L. Patricia C. Morellato
Departamento de Botânica, Plant Phenology and seed Dispersal Research Group, Universidade Estadual Paulista, C.P. 199, Cap. 13506-900 Rio Claro, São Paulo. Brasil. Fax: 55/19/35264201; igajardo@hotmail.com; pmorella@rc.unesp.br
Received 28-VIII-2001. Corrected 25-IX-2002. Accepted 16-XI-2002.
Abstract
The reproductive phenology of seven species of Rubiaceae from the Brazilian Atlantic rain forest was compared to evaluate the occurrence of phylogenetic constraints on flowering and fruiting phenologies. Since phenological patterns can be affected by phylogenetic constraints, we expected that reproductive phenology would be similar among plants within a family or genus, occurring during the same time (or season) of the year. Observations on flowering and fruiting phenology were carried out monthly, from December 1996 to January 1998, at Núcleo Picinguaba, Parque Estadual da Serra do Mar, Ubatuba, São Paulo State, Brazil. Nine phenological variables were calculated to characterize, quantify and compare the reproductive phenology of the Rubiaceae species. The flowering patterns were different among the seven species studied, and the Kruskal-Wallis test indicated significant differences in flowering duration, first flowering, peak flowering and flowering synchrony. The peaks and patterns of fruiting intensity were different among the Rubiaceae species studied and they differed significantly from conspecifics in the phenological variables fruiting duration, fruiting peak date, and fruiting synchrony (Kruskal-Wallis test). Therefore, we found no evidence supporting the phylogenetic hypotheses, and climate does not seem to constrain flowering and fruiting patterns of the Rubiaceae species in the understory of the Atlantic forest.
Key words: phenology, synchrony, flowering, fruiting, Rubiaceae, Atlantic Forest, Psychotria.
Phenological studies of tropical forests have traditionally focused on diverse taxonomic groups of species, investigating how climate seasonality and biotic interactions affect the plant phenological patterns observed (Frankie et al. 1974, Opler et al. 1976, 1980, Hilty 1980, Koptur et al. 1988, Schaik et al. 1993, Smith-Ramírez and Armesto 1994, Morellato et al. 1989, 2000, Brenes and DStefano 2001, Parolin 2002). However, some authors have considered non-adaptive hypotheses to explain plant phenology including endogenous factors, the plant life-history, and phylogenetic limitations to phenological responses (Borchert 1983, Eriksson et al. 1983, Kochmer and Handel 1986).
The study of reproductive phenological patterns within the same genus or family allow us to evaluate the importance of climatic seasonality and ecological interactions as selective forces relative to phylogenetic constraints (Kochmer and Handel 1986, Smith-Ramírez et al. 1998). Since phenological patterns may be affected by phylogenetic constraints, it would be expected that in plants within a family or genus, the reproductive phenology will be similar, occurring during the same time (or season) of the year (Rathcke and Lacey 1985, Kochmer and Handel 1986, Herrera 1992). We evaluated those assumptions for seven sympatric species of Rubiaceae in the understory of the Brazilian Atlantic rain forest. Based on the definition of several phenological variables we compared the reproductive phenology of those species and evaluated the existence of phylogenetic constraints.
Materials and methods
Study site: The study was carried out at Núcleo Picinguaba, Serra do Mar State Park, Ubatuba Municipality, São Paulo State, Brazil (23º 20- 23º 22S; 44º 46- 44º 51W). The general climate is tropical-wet or tropical rainy according to Köppen (1948) system, with substantial rain occurring every month. The mean annual rainfall and temperature at Ubatuba is 2 634 mm and 21.9ºC (1961-1990), with high mean temperatures in February (30.4ºC) and lowest in July (12.6ºC). During the wetter season, from October to April, monthly total rain-fall average is above 200 mm, and around 110 mm during the less wet or drier period, from May to September (Morellato et al. 2000). The shortest days occur in the beginning of the driest season. During this study, from December/96 to January/98, the mean temperature was 23.3ºC and total precipitation was 2 758 mm. November was the rainiest month (497.4 mm) and July the driest one (30.8 mm). Climate data are from the Estação Experimental de Ubatuba, Instituto Agronômico de Campinas (IAC).
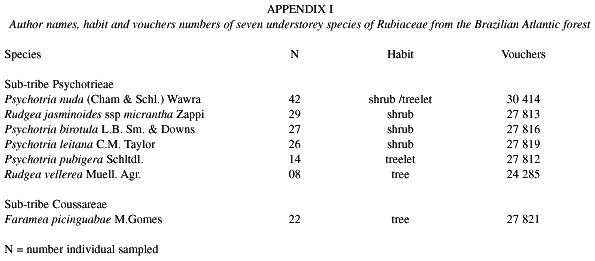
The vegetation at the study site is classified as pre-montane forest, low montane forest or simply Atlantic rain forest, and is considered to be a "typical" Atlantic forest (Sanchez et al. 1999, Oliveira-Filho and Fontes 2000). The irregular canopy is composed of trees 15 –25 m high, and includes a high diversity of species. A detailed description of the vegetation and climate can be found in Sanchez et al. (1999) and Morellato et al. (2000). We analyzed the phenology of 168 individuals distributed in seven Rubiaceae species (see Appendix I), sampled within 52 plots (10x10 m) distributed in well-preserved Atlantic rain forest (SanMartin-Gajardo and Morellato 2003). Vouchers of all studied species are deposited at the Herbarium Rioclarense (HRCB).
Plant Phenology: Observations on flowering and fruiting phenology were carried out monthly, from December 1996 to January 1998. Flowering was defined as presence of flower buds and/or open flowers since it is a characteristic of Rubiaceae species to produce many flower buds opening successively over a long period (Robbrecht 1988), and the phenological pattern was similar between flower buds and open flowers (SanMartin-Gajardo and Morellato 2003). For the same reasons, fruiting was also defined as the presence of unripe and ripe fruits. We applied the Fournier (1974) method to score the intensity of phenological events and to calculate the monthly percentage of activity for each species (SanMartin-Gajardo and Morellato 2003).
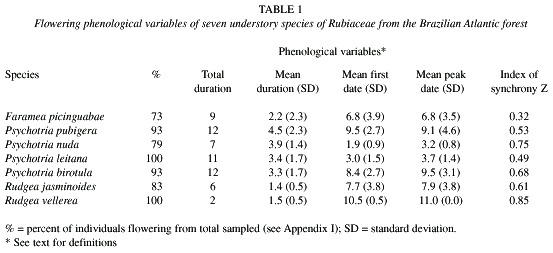
Phenological variables: In order to characterize, quantify and compare the reproductive phenology of Rubiaceae species nine phenological variables were calculated at individual (variables i, iv, vi, viii) and at population (variables ii, iii, v, vii, ix) levels (Augspurger 1985, Morellato et al. 2000) as follows:
i) Duration – number of months each individual spent flowering/fruiting.
ii) Mean duration – mean time, in months, a species spent flowering/fruiting, corresponding to the duration of the phenophase of each individual divided by the total number of individuals in that species.
iii) Total duration - the total number of months a species spent flowering/fruiting.
iv) Date of first flowering/fruiting – first month an individual began to flower/fruit. The date corresponding to the month in which the phenological event occurred. For statistical analyses, dates were converted into single numerical variables, and for each month was assigned a number from 1 to 12, starting with January.
v) First date synchrony – standard deviation around the mean of the dates of first flowering/ fruiting of each individual for a given species.
vi) Date of peak flowering/fruiting – month of maximum intensity of flowering/fruiting for an individual according to the Fournier index.
vii) Peak date synchrony – standard deviation around the mean of the dates of flowering/ fruiting peak of each individual of a given species. For variables v and vii, high standard deviation values indicate low synchrony among individuals of a given species and zero indicates maximum synchrony (see Augspurger 1983).
viii) Index of synchrony of a given individual with its conspecifics (X i) is defined as (Augspurger 1983):
Where ij is the number of months both individuals i and j are flowering/fruiting synchronously, j ?i; fi is the number of months the individual i is flowering, and N is the number of individuals in population. When X = 1, it perfect synchrony occurs or there is a complete overlap between flowering/fruiting periods of individual i and j, in the population; when X = 0, no synchrony occurs or there is no overlap between flowering/fruiting periods of the individuals i and j.
ix) Index of population Synchrony (Z) – estimates the overlap on flowering or fruiting periods among individuals of the same species, and is defined as (Augspurger 1983):
Z = Σ X1 / N
Where N is the number of individuals in population, and X i is the index of synchrony for individual i. When Z = 1, it indicates perfect synchrony or flowering/fruiting periods of all individuals in the population occurs in the same time of the year; when Z = 0, indicates no synchrony or there is no overlap on flowering/ fruiting periods among all individuals in the population/species.
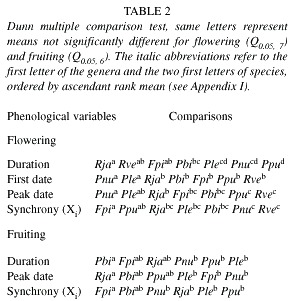
The phenological variables were calculated for each individual and for those species for which at least five individuals flowering or fruiting during the observations. As most of the data distributions were not normal (Shapiro-Wilk W test), we performed the following analyses: The Kruskal-Wallis test was applied to verify if there is a significant difference (or not) between phenological variables of each species. The phenological variables compared were duration, date of first flowering/fruiting, date of peak flowering/fruiting and synchrony (Xi ). When the Kruskal-Wallis test detected a significant difference, the Dunn test was performed for comparison between pairs of species. All analyses performed followed Zar (1996).
Results
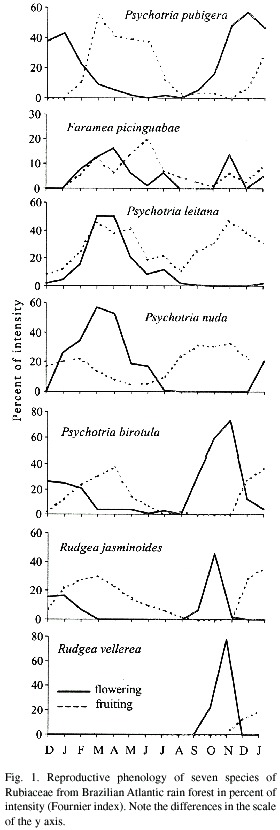
Flowering phenological variables: The percentage of individuals flowering per species was high, ranging from 73 to 100% (Table 1), and the flowering percent of Fournier was always above 50% for all species, except Faramea picinguabae (Fig. 1). The flowering patterns were different among the seven species observed, even though all species displayed a flowering peak during the wettest season, from October to March (Fig. 1). The Kruskal-Wallis test indicated significant differences in flowering duration (H=60.53, p< .001), first flowering (H=76.57, p< .001), peak flowering (H=64.89, p< .001) and flowering synchrony (H=50.69, p< .001) among the seven species studied. The pairwise comparisons for duration and peak date showed that the differences among the seven species studied were unable to distinguish groups of species (Table 2). However, the pair-wise comparisons for first date of flowering grouped Psychotria nuda and P. leitana against the other five species of Rubiaceae (Table 2). Those two species presented first flowering dates at the end of the rainy season, while the other five species started to flower in the beginning of the rainy season (Fig. 1).
The total flowering duration and mean flowering duration differed greatly among the studied species, with the total duration ranging from two to 12 months (Table 1). The high values of standard deviation around the mean of flowering duration indicate a strong variation at the individual level, mainly in F. picinguabae (Table 1). Only Rudgea vellerea and P. nuda presented low standard deviations around the mean of both the first day and the peak of flowering, indicating a high synchrony in these variables. The latter is confirmed by the high population index of synchrony (Z) (Table 1).
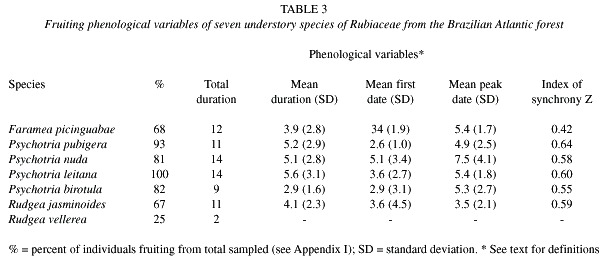
Fruiting phenological variables: The percentage of individuals fruiting per species was high, ranging from 67 to 100% (Table 3). The only exception was R. vellerea with two out of eight flowering individuals producing fruits (Table 3). The fruiting percentage of Fournier was around 30% for most of the species, and both, the peaks and patterns of fruiting intensity, were different among the species (Fig. 1). In fact, the six species differed significantly from conspecifics in the duration of each phenophase (H=14.73, p< .01), the peak date (H=11.86, p< .05), and the synchrony (H=20.68, p< .001), but the pairwise comparisons performed for these three phenological variables were unable to distinguish groups of species (Table 2). Only the first date of fruiting did not differ significantly among the species.
The six species displayed low individual synchrony (high values of standard deviation) for mean fruiting duration, mean fruiting first date, and mean fruiting peak date (Table 3). This trend is confirmed by the low values of the population index of synchrony (Z), reaching up to 0.64 (Table 3). The total fruiting duration was long, ranging from nine months in P. birotula to 14 months in P. leitana and P. nuda.
Discussion
The flowering patterns were different among the seven species of Rubiaceae studied at Picinguaba, in the Atlantic rain forest, with significant differences in flowering duration, first flowering, peak flowering and flowering synchrony. The peaks and patterns of fruiting intensity were also different among the Rubiaceae species and they differed significantly in the phenological variables such as fruiting duration, fruiting peak date, and fruiting synchrony. Similar reproductive phenology, occurring in the same time or season of the year, expected for species within the same family (cf. Kochmer and Handel 1986), was not observed for most of the phenological variables analyzed. Therefore, we found no evidence supporting the phylogenetic hypotheses, as suggested by Kochmer and Handel (1986).
Differences on flowering phenology among species within the same family, described here for Rubiaceae, were also observed for Bignoniaceae (Gentry 1974), Boraginaceae (Opler et al. 1975), Piperaceae (Fleming 1985), and Lauraceae (Wheelwright 1985). The general interpretation has been the sharing pollinator hypothesis: the interspecific competition for pollinators been a selective pressure leading to a segregation on flowering episodes among species (Ratchke and Lacey 1985). However, our knowledge of pollination ecology of Atlantic forest Rubiaceae is not great enough to evaluate this hypothesis. In the same way, the occurrence of Rubiaceae species fruiting all year round could be interpreted as a pattern defined by interspecific competition for seed dispersers (see Wheelwright 1985, Skeate 1987, Gorchov 1990). However, the Rubiaceae at Picinguaba does not fit in the description of sequential fruiting, since all species presented long or continuous fruiting periods with different degrees of overlap among them.
The differences among the Rubiaceae species studied are better understood when we analyze the phenological variables at an individual level (duration, date of first flowering/fruiting, peak flowering/fruiting and index of synchrony), and the population index of synchrony (Z). The flowering index of synchrony was significantly different among the seven species of Rubiaceae studied. Similar results are described by Augspurger (1983) for shrubs from seasonal forest at Panama. Both studies report a large range of Z values, from synchronous to asynchronous species. Augspurger (1983) correlated high flowering synchrony with short flowering duration and high species density in four of six species studied, including one Rubiaceae, P. horizontalis (Z = 0.85). Similar correlations (Pearson test p< .05) were not found for R. vellerea, P. nuda and P. birotula, the species with highest index of synchrony (Z > 0.68) in this study. Medium to low values of reproductive synchrony (Z), presented by most of the Rubiaceae species studied, are usually described for treelet and shrubs from the understory of tropical forests (Opler et al. 1980). The low synchrony observed is related to the long duration of reproductive phases, another pattern described for species from the understory of tropical forests (Frankie et al. 1974, Opler et al. 1975, Hilty 1980, Rathcke and Lacey 1985, Koptur et al. 1988, Levey 1988, Marquis 1988). Among the explanations for low synchrony we consider the inconstancy of selective forces and/or environmental heterogeneity (Ollerton and Lark 1992, 1998, Fox and Kelly 1993) the better interpretation for the patterns observed for Atlantic forest Rubiaceae.
Consequently, there does not seem to exist a strong environmental selective pressure constraint for the time of flowering or fruiting of the species studied. In fact, due to the low climate seasonality at the study site (see Morellato et al. 2000), the climatic factors also do not seem to limit the reproductive phenology of Rubiaceae species from the understory of the Atlantic forest, allowing flowering and fruiting to occur at any time of the year.
Acknowledgments
The authors thank M. A. Pizo for critical reading of the manuscript, M. Gomes, S. Jung-Mendoçali and D. Zappi for species identification, C. C. Castro for allowing access to unpublished data, Allen H. Fetter for English review, and the Instituto Florestal for making possible our work at Núcleo Picinguaba, Parque Estadual da Serra do Mar. Financial support for field work was provided by Coordenadoria de Aperfeiçoamento de Pessoal e Ensino Superior (CAPES), and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP #95/9626-0). Fellowships for I.S.M.G. (#830818/97-9) and L.P.C.M (#301602/91-3) were provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Resumen
La fenología reproductiva de siete especies de Rubiaceae de una selva pluvial tropical Atlántica fue comparada para avaluar la presencia de limitaciones filogenéticas en los patrones de floración y fructificación. Dado que los patrones fenológicos puedan ser afectados por limitaciones filogenéticas, nosotros esperamos que la fenología reproductiva podría ser similar en plantas de una familia o género, en la misma estación durante el año. Las observaciones de la fenología de floración y fructificación fueron hechas mensualmente, de diciembre de 1996 a enero de 1998, en el Núcleo Picinguaba, Parque Estadual da Serra do Mar, Ubatuba, São Paulo, Brasil. Nueve variables fenológicas fueron calculadas para caracterizar, cuantificar y comparar la fenología reproductiva de las especies de Rubiaceae. Los patrones de floración fueron diferentes entre las siete especies, el análisis de Kruskall Wallis indicó diferencias significativas en la duración, fecha de inicio, fecha de producción máxima y sincronía de floración. Los patrones de fructificación fueron diferentes entre las especies estudiadas y fueron también significativamente diferentes en las variables fenológicas: duración, fecha de producción máxima y sincronía de fructificación. Por lo tanto, nosotros no encontramos evidencias para apoyar la hipótesis filogenética y el clima parece no ser un fuerte limitante en los patrones de floración y fructificación de las especies estudiadas en el sotobosque de la floresta Atlántica.
References
Augspurger, C.K. 1983. Phenology, flowering synchrony, and fruit set of six neotropical shrubs. Biotropica 15: 257-267. [ Links ]
Augspurger, C.K. 1985. Flowering synchrony of neotropical plants, pp. 394-410. In W. DArcy & M. D. Correa (eds.). The botany and natural history of Panama. Missouri Botanical Garden, Saint Louis, Missouri. [ Links ]
Borchert, R. 1983. Phenology and control of flowering in tropical trees. Biotropica 15: 81-89. [ Links ]
Brenes C., L. & J.F. DStefano. 2001. Compartamiento fenológico del árbol Elaeagia uxpanapensis (Rubiaceae), en un bosque pluvial premontano de Costa Rica. Rev. Biol. Trop. 49: 989-998. [ Links ]
Eriksson, O., O. Inghe, L. Jerling, P.G. Tapper, A. Telenius & P. Torstensson. 1983. A note on non-adaptation hypotheses in plant ecology. Oikos 41: 155-156. [ Links ]
Fleming, T.H. 1985. Coexistence of five sympatric Piper (Piperaceae) species in a tropical dry forest. Ecology 66: 688-700. [ Links ]
Fournier, L.A. 1974. Un método cuantitativo para la medición de características fenológicas en árboles. Turrialba 24: 422-423. [ Links ]
Fox, A.G. & K.C. Kelly. 1993. Plant Phenology: Selection and Neutrality. TREE 8: 34-35. [ Links ]
Frankie, G.W., H.G. Baker & P.A. Opler. 1974. Comparative phenological studies of trees in tropical wet and dry forest in the lowland of Costa Rica. J. Ecol. 62: 881-919. [ Links ]
Gentry, A.H. 1974. Flowering phenology and diversity in tropical Bignoniaceae. Biotropica 6: 64-68. [ Links ]
Gorchov, D.L. 1990. Pattern, adaptation, and constraint in fruiting synchrony within vertebrate-dispersal wood plants. Oikos 58: 169-180. [ Links ]
Herrera, C. 1992. Interespecific variation in fruit shape: allometry, phylogeny, and adaptation to dispersal agents. Ecology 73: 1832-1841. [ Links ]
Hilty, S.L. 1980. Flowering and fruiting periodicity in a premontane rain forest in Pacific Colombia. Biotropica 12: 298-306. [ Links ]
Kochmer, J.P. & S. Handel. 1986. Constraints and competition in the evolution of flowering phenology. Ecol. Monog. 56: 303-325. [ Links ]
Koeppen, W. 1948. Climatologia. Fondo de Cultura Económica, México, México. 213 p. [ Links ]
Koptur, S., W.A. Haber, G.W. Frankie & H.G. Baker. 1988. Phenological studies of shrub and treelet species in tropical cloud forest of Costa Rica. J. Trop. Ecol. 4: 323-346. [ Links ]
Levey, D.J. 1988. Spatial and temporal variation in Costa Rican fruit and fruit-eating birds abundance. Ecol. Monog. 58: 251-269. [ Links ]
Marquis, R.J. 1988. Phenological variation in the neotropical understory shrub Piper arieianum: causes and consequences. Ecology 69: 1552-1565. [ Links ]
Morellato, L.P.C., R.R. Rodrigues, H.F. Leitão-Filho & C.A Joly. 1989. Estudo comparativo da fenologia de espécies arbóreas de floresta de altitude e floresta mesófila semidecídua na Serra do Japi, Jundiaí, São Paulo. Revta. Brasil. Bot. 12: 85-98. [ Links ]
Morellato, L.P.C., D.C. Talora, A. Takahasi, C.C. Bencke, E.C. Romera & V.B. Zipparro. 2000. Phenology of Atlantic Rain Forest trees: a comparative study. Biotropica 32: 811-823. [ Links ]
Oliveira-Filho, A.T. & M.A. Fontes. 2000. Patterns of floristic differentiation among Atlantic forests in South-eastern Brazil, and the influence of climate. Biotropica 32: 793-810. [ Links ]
Ollerton, J. & A.J. Lack. 1992. Flowering phenology: An example of relaxation of natural selection? TREE 7: 274-276. [ Links ]
Ollerton, J. & A.J. Lack. 1998. Relationships between flowering phenology, plant size and reproductive success in Lotus corniculatus (Fabaceae). Plant Ecology 139: 35-47. [ Links ]
Opler, P.A., G.W. Frankie & H. G. Baker. 1976. Rainfall as a factor in the release, timing, and synchronization of anthesis by tropical trees and shrubs. J. Biogeog. 3: 231-236. [ Links ]
Opler, P.A., H.G. Baker & G.W. Frankie. 1975. Reproductive biology of some Costa Rica Cordia species (Boraginaceae). Biotropica 7: 234-247. [ Links ]
Opler, P.A., G.W. Frankie & H.G. Baker. 1980. Comparative phenological studies of treelet and shrub species in tropical wet and dry forests in the lowlands of Costa Rica. J. Ecol. 68: 167-188. [ Links ]
Parolin, P. 2002. Life history and environment of Cecropia latiloba in Amazonian floodplains. Rev. Biol. Trop. 50: 531-545. [ Links ]
Rathcke, B. & E.P. Lacey. 1985. Phenological patterns of terrestrial plants. Ann. Rev. Ecol. Syst. 16: 179-214. [ Links ]
Robbrecht, E. 1988. Tropical woody Rubiaceae: characteristic features and progression contributions to a new subfamilial classification. Nationale Plantentuin van Belgie, Meise. 271 p. [ Links ]
Sanchez, M., F. Pedroni, H.F. Leitão-Filho & O. Cesar. 1999. Composição florística de um trecho de floresta ripária na Mata Atlântica em Picinguaba, Ubatuba, SP. Rev. Brasil. Bot. 22: 31-42. [ Links ]
SanMartin-Gajardo I. & L.P.C. Morellato. 2003. Fenologia de Rubiaceae do sub-bosque em floresta Atlântica no sudeste do Brasil. Rev. Brasil. Bot. 26: 299-309. [ Links ]
Schaik, C.P. van, J.W. Terborgh & S.J. Wright. 1993. The phenological of tropical forest: adaptive significance and consequences for primary consumers. Ann. Rev. Ecol. Syst. 24: 353-377. [ Links ]
Skeate, S.T. 1987. Interactions between birds and fruits in a northern Florida hammock community. Ecology 68: 297-309. [ Links ]
Smith-Ramírez, C., J.J. Armesto & J. Figueroa. 1998. Flowering, fruiting and seed germination in Chilean rain forest Myrtaceae: ecological and phylogenetic constraints. Plant Ecology 136: 119-131. [ Links ]
Smith-Ramírez, C. & J.J. Armesto. 1994. Flowering and fruiting patterns in the temperate rainforest of Chiloé, Chile: ecologies and climatic constraints. J. Ecol. 82: 353-365. [ Links ]
Wheelwright, N.T. 1985. Competition for dispersers, and the timing of flowering and fruiting in a guild of tropical trees. Oikos 44: 465-477. [ Links ]
Zar, J.H. 1996. Bioestatistical analysis. Prentice-Hall, New Jersey. 662 p. [ Links ]