Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.49 n.2 San José Jun. 2001
Tortricidae) oviposition on macadamia nuts
Helga Blanco-Metzler1 Allan D.Watt2 Derek Cosens3
Recibido 22-II-2000. Corregido 27-IX-2000. Aceptado 17-I-2000.
Abstract
Vertical distribution of eggs of the macadamia nutborer Ecdytolopha torticornis Meyrick (Lepidoptera: Tortricidae) and its preference of oviposition sites within and between macadamia cultivars were studied in Turrialba, Cartago, Costa Rica, in 1992 (N = 6 939). E. torticornis eggs were found throughout the foliar parts of the tree, but fewer eggs were laid in the crown top than in the mid or lower crown. Differences in the horizontal distribution of the eggs were not significant, albeit more eggs were found in the outer positions. The numbers of eggs found within the crowns of different clones were similar, implying that the nutborer has no preference for a particular cultivar.
Key words
Ecdytolopha torticornis, oviposition, sampling, macadamia, Costa Rica.
Since it was first recorded in Costa Rica (F. Lara 1987 unpublished) the nutborer Ecdytolopha torticornis Meyrick (Lepidoptera: Tortricidae), has been responsible for an increase in damage to the nuts of Macadamia integrifolia Maiden and Betche (Proteaceae) from a loss in yield of 16 % in 1987 (Lara 1987), to 28% in 1990 (C.E Masís and L.F Campos 1990 unpublished), and 39 % in 1992 (Blanco-Metzler et al. 1994).
The E. torticornis female lays its eggs singly on the middle zone of macadamia nuts and, usually, in the narrow space between adjacent nuts within a cluster. Upon emergence the larva bores into the nut and feeds on the husk, if the nut shell has not hardened the larva will bore through, and feed on the kernel (Blanco et al. 1993). The biology of E. torticornis closely resembles that of the koa seedworm Cryptophlebia illepida (Butler) (Lepidoptera: Tortricidae), and the litchi fruit moth, C. ombrodelta (Lower) (Jones and Caprio 1992). E. torticornis moths have been reported to be poor fliers (Chamberlain 1989) living in the tree canopy (Blanco-Metzler 1994), and since the larvae feed inside the nut, they are limited in their ability to disperse. Thus, the distribution of larvae and hence nut damage are largely determined by egg distribution.
The oviposition behaviour of the nutborer within the host plant has provided useful and necessary information for the development of sampling methods. A survey of total egg mass is one of the methods used in determining pest abundance in many horticulturas crops as Diaphania nitidalis (Stoll) (Lepidoptera: Pyralidae) in cucumber (Blanco-Metzler 1983); Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) in maize (Hoffmann et al. 199l), and in forest pests such as the gypsy moth, Lymantria dispar (L.) (Lepidoptera: Lymantriidae) in birch trees (Higashiura 1989, Thorpe and Ridgway 1992) or the spruce budworm, Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae) in spruce trees (Lysyk 1990). The aim of this study was to investigate the within-tree distribution of the nutborer by recording its oviposition sites, and to determine whether moths showed ovipositional preferences between cultivars.
Materials and Methods
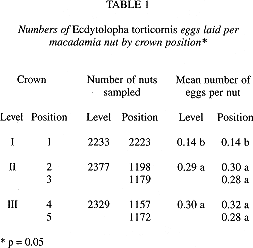
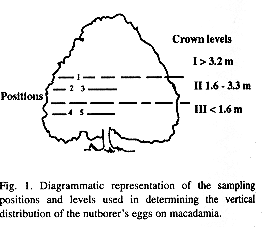
The study site was located at Oriented Farm, Turrialba, Costa Rica, at elevations ranging from 620 to 700 m above sea leves. The vertical distribution of E. torticornis eggs was studied in a mixed stand planted in 1978 of four of the most commonly planted cultivars in Costa Rica: Keahou (HAES 246), Kau (HAES 344), Kakea (HAES 508) and Keaau (HAES 660) with a height ranging from 4.5 m to 6 m. The study period was from July to December 1992. The nuts were examined in the laboratory (23°C, 80% RH) at CATIE (Tropical Agronomical Research and Higher Education Centre, Turrialba), and the following variables were evaluated: the number of eggs per nut; the number of fertile and infertile eggs and empty chorions; and the number of nutborer holes per nut. The number of holes was included as an indication of multiple, successful ovipositions. Some nuts were found to have holes but no chorions were present perhaps because they had been disloged by rain, blown away by wind, or eaten by predators. The same trees were sampled every month. Trees were divided into tree crown levels (Fig. 1): I, crown top, 3.2 m from the ground to the top of the tree crown position l); II, mid-crown, 1.6 - 3.2 m from the ground (positions 2 and 3); III, lower-crown, 0 - 1.6 m from the ground (positions 4 and 5). The height of level III corresponded to that of the first author. Levels II and III were each divided into two positions: positions 3 and 5 = nuts sampled in an area up to 1 m horizontally from the tree trunk; posidons 2 and 4 = nuts sampled in an area up to 1 m horizontally from the end of the branches. Twenty nuts (over 0.8 cm in diameter) per crown level were collected at random; ten nuts per position for crown levels II and III.
The data were submitted to Analysis of Variance and T tests (LSD) (Anonymous, 1985). The experimental design used was a split split plot where the tree was the big plot, the combination of levels and positions was the split plot and the time (6 months, July to December) was the split split plot. Data were transformed by sqrt (x + 0.5) as many nuts were free of eggs. Nuts were collected every month from five trees per clone, 20 trees in total. In the statistical analysis, crown level I was compared with levels II and III together; level II versus level III; and the inner sampling positions (3 and 5) were compared with the outer sampling positions (2 and 4).
Results
A total of 6 939 nuts were inspected. Newly laid eggs were frequently observed on nuts already bearing eggs or bore-holes. The number of eggs laid per nut varied from cero to eight with a mean of 0.8 (n = 201 nuts). No significant differences were found in the number of eggs per cultivar (F = 0.69; d.f. = 3; p = 0.57).
Eggs were found on macadamia trees at all heights; however, they were significantly more abundant in crown levels II and III than in level I (p < 0.001). The mean number of eggs per nut in top level I was 0. 14; in mid level II: 0.29; and in bottom level III: 0.30. There was no significant difference between the number of eggs found on nuts from the lower two levels (F = 0.22; d.f. = 1; p = 0.64); nor between the mean number of eggs found on nuts on the inner and outer parts of the branches (F = 2.0; d.f. = 1; p = 0. 16) (Table 1), While fewer eggs were found on the inner sampling positions (3 and 5) than on the outer sampling positions (2 and 4), no statistical difference was found between the number of eggs in either positions for levels II and III (F = 0.22; g.1. = 1; p = 0.64).
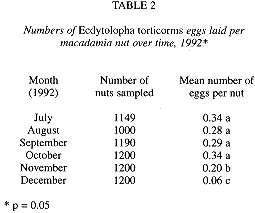
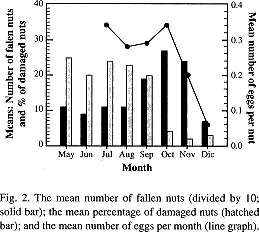
There was a significant difference (p < 0.0001) in the number of eggs per nut over the six months of sampling (Table 2). The number of eggs per nut was highest from July to October, decreased in November (0.2) and dropped steeply to a minimum (0.06) in December (Fig. 2). The decline in the number of eggs per nut paralleled the decline in nut production, but not the decline in the number of damaged nuts which occurred approximately one month earlier (Fig 2).
Discussion
Oviposition site selection is one of the most important aspects of habitat selection in insects. It varies with insect species and is closely related to offspring survival. The observed habit of laying eggs on nuts
already containing eggs or on damaged nuts (Blanco-Metzler et. al. 1993) might give the larvae the advantage of escaping from parasitoids and predators, or prevent them from being dislodged by the rain. Blanco-Metzler (1994) reported that it took a larva approximately fifty minutes from emerging from the egg to completely bore through the husk. T'herefore, if an entrance to the nut has already been made, the first instar larva is more likely to evade detrimental enviromnental factors and increase its survivorship. Conversely, the females' habit of laying eggs on damaged nuts may lead to intra-specific larval competition for food and/or space. Moreover, larval mortality might increase due to early nut-drop (Blanco-Metzler et. al. 1992; Blanco-Metzler 1994). Sinclair (1979) observed that Cryptophlebia ombrodelta, also tended to lay its eggs preferentially on damaged macadamia nuts; he concluded that this behaviour lowered the chances of survival of the larvae in dense populations due to cannibalism and early nutfall. However, cannibalism was not observed in E. torticornis: yet early nut-fall was observed and concomitant enhanced larvas predation (Blanco-Metzler et al. in preparation).
Oviposition in insects has been reported to be influenced by physical properties of the host such as shape of the trees and nuts, colour of leaves and nuts, surface texture of fruits (Prokopy and Bush 1973) and chemical stimulants of the host (Renwick and Radke 1983; Hedin and McCarty 1990). Since no differences were found in the number of eggs between cultivars it is unlikely that during oviposition females were influenced by cultivar variation in shape, size, and colour of die nuts. Thus, the differences in nut damage for cultivars 246, 344, 508 and 660 (C.E. Masís and L.F. Campos 1990; Blanco-Metzler, et. al. 1992; Blanco-Metzler 1994) might rather reflect differences in larval survival resulting from cultivar differences in food quality due to variation in secondary plant compounds and husk toughness. Indeed, damage was found to decrease with nut maturation because as the nut dries it provides food of a poorer quality and quantity.
The tendency of finding most eggs in the outer bottom level of the tree crown, followed by the outer middle level, could be because nut production is highest in this part of the tree (Diego Pérez, personal communication), and therefore, the moths lay their eggs where food abundance is highest to ensure the best possible food supply for their offspring. Also, since E. torticornis has been reported as probably being a poor flyer (Chamberlain 1989), the adults reach this part of the tree more readily from the weeds where they are believed to feed. Thus during the period of oviposition, they do not have to fly far to find their host and lay their eggs. In Oriente Farm weed control is limited as they have found that weeding increases root fungi problems and soil erosion (Diego Pérez, personal communication).
There are three probable reasons that could explain the differences in the number of eggs per nut found over time. First, during the period September to November nut production was high and females found plenty of oviposition sites: as time passed and fewer nuts (oviposition sites) were available and, thus, were thus harder to find. Secondly, a reduction in the number of nuts restricts the available larval food resource and in turn the larvas population, hence fewer adults will eventually emerge. Thirdly, the seasonal increase in the abundance of parasitoids (Blanco-Metzler 1994) is also likely to cause a decline in the size of the nutborer population.
Although Cryptophlebia females show no preference for laying eggs at different heights in the tree crowns (Jones and Tome 1992) E. torticornis females do prefer to oviposit on nuts growing within the first tree metres of the ground. We reaffirm comments by Southwood (1978) and Horn (1988) that the identification of host characteristics relevant to oviposition behaviour and insect distribution (both within and between host plants) is important for the design of sampling programmes, the forecasting of insect damage, and for assessment of the effectiveness of control programmes.
Aknowledgements
This research was supported by theBritish Council, AID-ROCAP, the Tropical Agronomical Research and Higher Education Centre (CATIE) and Macadamia de Costa Rica.
Resumen
Se determinó la distribución vertical de los huevos del barrenador de la nuez de macadamia Ecdytolopha torticornis Meyrick (Lepidoptera: Tortricidae) y los sitios de preferencia de oviposición en los árboles y entre clones de macadamia. Se detectó la presencia de huevos de E. torticornis en todo el árbol, sin embargo, se encontró un menor número de huevos en la parte alta de la corona que en la parte media e inferior. La diferencia en la distribución horizontal de los huevos fue no significativa, a pesar de encontrarse un mayor número de huevos en las posiciones externas. El número de huevos entre clones fue similar, sugiriendo que la polilla del barrenador no tiene preferencias de oviposición entre clones.
References
Anonymous. 1985. SAS user's guide: statistics, version 5. l. Cary, North Carolina, SAS Instituto. [ Links ]
Blanco-Metzier, H. 1983. Distribución de oviposición de Diaphania nitidalis (Stoll) y D. hyalinata (L.)
(Lepidoptera. Pyralidae) en pepino. Ing. Agr. Thesis. University of Costa Rica, San José. 35 p.
Blanco-Metzler, H. 1994. The biology and ecology of the macadamia nutborer, Ecdytolopha torticornis
Meyrick (Lepidoptera: Tortricidae) in Costa Rica. Ph.D. Thesis, University of Edinburgh, Scotland. 131 p.
Blanco-Metzler, H., A.D Watt & Cosens, D. 1992. Dynamics of macadamia nut damage by Ecdytolopha
torticornis Meyrick (Lep: Tortricidae) and parasitism by Apanteles spp. In Individuals, Patterns, and
Populations (Norwich, England, 7- 10 September, 1992).
Blanco-Metzler, H., A.D Watt & Cosens, D. 1993. Ciclo de vida y comportamiento de oviposición de Ecdytolophatorticornis Meyrick (Lepidoptera: Tortricidae), barrenador de la nuez de macadamia. Rev. Manejo Integr. Plagas (Costa Rica) No. 29: 36-39. [ Links ]
Chamberlain, D.J. 1989. Fieid studies on the distribution and damage attributable to a macadamia nutborer, (Ecdylolopha torticornis Meyrick). In D. Chamberlain, P.S. Beevor & A. Cork (eds.). Preliminary investigations on the biology and pheromone identification of a new nutborer pest of macadamia in Costa
Rica conducted in 1989. Overseas Development Natural Resources Institute, Kent, U.K.
Hedin, P.A., & J.C. McCarty. 1990. Possible roles of cotton bud sugars and terpenoids in oviposition by the boll weevil. J. Chem. Ecol. 16: 757-722. [ Links ]
Higashiura, Y. 1989. Survival of eggs in the gypsy moth Lymantria dispar. II. Oviposition site selection in changing environments. J. Anim. Ecol. 58: 413-426. [ Links ]
Hoffmann, M., L.T. Wilson, F.G. Zalom & R.J. Hilton. 1991. Dynamic sequential sampling plan for Helicoverpa zea (Lep: Noctuidae) eggs in processing tomatoes: parasitism and temporal patterns. Env.Entomol. 20: 1005-1012. [ Links ]
Horn, D.J. 1988. Ecological approach to pest management. Guilford Press, New York. 285 p. [ Links ]
Jones, VP. & L.C. Caprio. 1992. Damage estimates and population trends of insects attacking seven macadamia cultivars in Hawaii. J. Econ. Entomol. 85: 1884-1890. [ Links ]
Jones, V.P. & C.H.M. Tome. 1992. Effect of husk feeding by Cryptophlebia spp. on macadamia in Hawaii. In Proccedings of the First International Macadanda Research Conference, July 28-30, 1992. Honolulu, University of Hawaii at Manoa. [ Links ]
Lysyk, T.J. 1990. Relationships between spruce budworm (Lep: Tortricidae) egg mass density and resultant defoliation of balsam fir and white spruce. Can. Entomol. 122: 253-262. [ Links ]
Prokopy, R.J. & G.L. Bush. 1973. Oviposition responses to different sizes of artificial fruit by flies of Rhagoletis pomonella species group. Ann. Entomol. Soc. Amer. 66: 927-929. [ Links ]
Renwick, J.A.A. & C.D. Radke. 1983 Chemical recognition of host plants for oviposition by the cabbage butterfly, Pieris rapae (Lepidoptera: Pieridae). Env. Entomol. 12: 446- 450. [ Links ]
Sinclair, E.R. 1979. Parasites of Cryptophlebia ombrodelta (Lower) (Lep: Tortricidae) in Southeast Queensland. J. Aust. Entomol. Soc. 18: 329-335. [ Links ]
Southwood, T.R.E. 1978. Ecological methods with particular references to the study of insect populations. 2nd. ed. London, Chapman and Hall. 524 p. [ Links ]
Thorpe, K.W. & R.L: Ridgway. 1992. Gypsy moth (Lep: Lymantriidae) egg mass distribution and sampling in a residential setting. Eny. Entomol. 21: 722-730. [ Links ]
1 Centro de Investigación en Protección de Cultivos-Estación Experimental Fabio Baudrit Moreno,
Universidad de Costa Rica, Facultad de Agronomía, San José, Costa Rica, Fax (506) 433-9086,
E-mail: hblanco@cariari.ucr.ac.cr
2 Banchory Research Station, Hill of Brathens, Glassel, Banchory AB31 4BY, Scotland, UK
E-mail: adw@wpo.nerc.ac.uk
3 Institute of Cell, Animal and Population Biology, The University of Edinburgh, Ashworth Laboratories,
West Mains Road, Edimburgh EH9 3JT, Scotland, UK E. Mail: Derek.Cosens@ed.ac.uk