Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Acta Médica Costarricense
On-line version ISSN 0001-6002Print version ISSN 0001-6012
Acta méd. costarric vol.63 n.3 San José Jul./Sep. 2021
http://dx.doi.org/10.51481/amc.v63i3.1132
Original
Bordetella bronchiseptica pneumonia in a patient with a history of pulmonary lymphoma
1 Caja Costarricense de Seguro Social, Hospital Tomás Casas Casajús, Laboratorio Clínico. Osa, Puntarenas. 0000-0001-7888-1926
2 Caja Costarricense de Seguro Social, Hospital Tomas Casas Casajús, Servicio de Medicina Interna. Osa, Puntarenas. 0000-0001-7721-8225
3 Caja Costarricense de Seguro Social, Hospital Tomás Casas Casajús, Laboratorio clínico. Osa, Puntarenas. 0000-0002-4216-8018
Bordetella bronchiseptica is a Gram-negative, pleomorphic, aerobic, coccobacillus, normal flora of the upper respiratory tract of domestic and wild mammals such as cats, dogs, pigs, rabbits, rodents, and seals, which it frequently causes respiratory infections. This bacterium is not part of the normal bacterial flora of humans.1-3
The genus Bordetella includes 9 species: B. pertussis, B. parapertussis, B. bronchiseptica, B. avium, B. holmesii, B. hinzii, B. trematum, B. petrii, and B. ansorpii, of which B. pertussis (causative agent of pertussis), B. parapertussis, and B. holmesii have been the most frequently associated with respiratory infections in humans.4-6
B. bronchiseptica infections in humans are rare, occurring mainly in immunocompromised individuals, and are considered an opportunistic infection.
Infection usually begins with adherence of the bacterium to the cilia of epithelial cells lining the upper respiratory tract. This adherence is facilitated by virulence factors present in the genus Bordetella (adhesins and autotransporters), so most infections correspond to the respiratory tract, where it has been found to cause sinusitis, bronchitis, tracheobronchitis, and pneumonia. However, it has also been isolated as a causative agent of endocarditis, sepsis, meningitis, peritonitis, and recurrent bacteremia.5,7
It has been suggested that the likely route of transmission is from small droplets of respiratory secretions during close contact with infected animals.1,3,8 Since it mainly affects immunocompromised individuals, the isolation of this bacterium in people with no known condition or disease affecting the immune system should raise a red flag and direct attention to the search for the cause of this decrease in the immune response.9
Case report
A 67-year-old woman, with a history of pulmonary lymphoma (discharged in 2004), with atelectasis, fibrosis, and right pulmonary bronchiectasis as sequelae of the treatments received (radiotherapy and chemotherapy), who also presents arterial hypertension and diabetes mellitus, consulted the emergency department of the Hospital Tomás Casas Casajús, after 5 days of increasing dyspnea, cough and phlegm.
On day 1 of admission she presented tachypnea and hypoxemia and was therefore treated as a suspected case of Covid19. Initial laboratory tests showed: O2 saturation 91%, hemoglobin 12.5 g/dL, hematocrit 36.9%, leukocyte count 14.9 x 103 (77% neutrophils and 15% lymphocytes), procalcitonin < 0.10 ng/mL, D-Dimer 0.91 mg/L. Molecular testing for SARS-CoV-2 was requested from the laboratory service. The sample, a nasal swab, was subjected to a molecular panel for the determination of viral and bacterial respiratory pathogens. The result was negative for all pathogens (SARS CoV- 2 and other respiratory viruses, Bordetella pertussi, B. parapertussi, Chlamydia pneumonidae, and Mycoplasma pneumonidae). After this result, on day 2, the patient was hospitalized for complicated bacterial pneumonia, a sputum sample was sent to the laboratory for bacterial culture and antibiotic coverage with cefotaxime was initiated. On day 5, a single bacterial isolate was obtained and identified as Bordetella bronchioseptica, using the VITEK 2® kit. The sensitivity test reported for this isolate reflected a MIC >= 64 for cefotaxime. Given this result and the poor improvement of dyspnea and cough, it was decided to change the antimicrobial therapy to intravenous gentamicin for 7 days with which an improvement of respiratory symptoms was achieved. At the same time, the isolate was sent to the National Bacteriology Reference Center of INCIENSA, where the MALDI-TOF MS mass spectrometry technique confirmed the bacterial identification of Bordetella brochioseptica and the E-test method confirmed resistance to cefotaxime.
Also, on day 5 a CT scan of the chest was performed (Figure 1) in which tissue retraction, atelectases, and fibrosis in the right lung field were documented, which were already considered sequelae of the treatment received against the lymphoma.
Additionally, diffuse infiltrates with a tendency to consolidate and two masses in the left upper lobe were observed. An evaluation was requested by the Pneumology Service of the referral hospital, and, for this purpose, the patient attended on the 14th and 19th days to this other hospital center of greater complexity, where she underwent a bronchoscopy and bronchial aspirates to be analyzed for pyogens and fungi, both in the right and left lower lobes. The bronchoscopy showed scant mucoid material in the glottis, but no lesions in the vocal cords, laryngeal mucosa, trachea, main carina, and left and right bronchial tree. Bacterial cultures were negative for mycobacteria and fungi, with the growth of normal upper tract flora, which evidenced the effectiveness of antimicrobial therapy. Multiple biopsies of the left upper lobe were also performed under fluoroscopic guidance, in the lingular segments, with mild lymphocytic inflammatory infiltrate and edema, which were negative for malignancy.
As part of the pandemic care controls, a second sample was retested for SARS-CoV-2 by a PCR determination and on day 20 a positive result was received with a cycle threshold (CT) of 33, interpreted as a low viral load and possible recent infection. However, given the high oxygen requirements that the patient began to present, she was transferred to a higher complexity hospital where she died on day 23, due to respiratory complications related to Covid19.
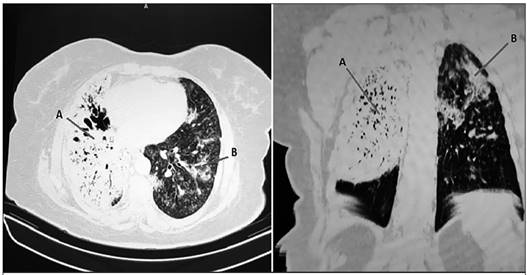
Figure 1 Chest CT image with contrast medium, showing A. tissue retraction in the right lung field, atelectases, and, significant fibrosis; B. in the left lung field diffuse infiltrates with a tendency to consolidation and two masses in the left upper lobe.
Most Bordetella bronchiseptica infections documented in humans correspond to patients with a decreased immune system response caused by a pre-existing disease or because of immunosuppressive therapy.2,11,12 In this case the patient presents chronic immunosuppression as a consequence of chemotherapy and radiotherapy received 16 years ago against pulmonary lymphoma.
Although little is known about the mechanisms of human-to-human transmission of B. bronchiseptica, cases have been reported in which transmission occurs by droplets expelled from the nose or mouth of an infected person when coughing, sneezing, or talking. 13 This reinforces the need to protect the immunosuppressed population by isolation or at least with the use of masks.
In this case, although the bacterial infection was effectively controlled, the patient suffered a Covid19 coinfection. It has been described that in patients with Covid19 and bacterial coinfection there is a decrease in the activation of the host defense, which may result in increased susceptibility to virus infection and the development of the pathology.
Additionally, the production of interferon I and III in bacterial infections stimulates the expression of the receptor for angiotensin-converting enzyme 2 (ACE2), which is used by SARS-CoV-2 to enter human cells. This increase in ACE2 expression, in addition to enhancing virus infection, has been associated with more severe cases of Covid19.14,15 It is difficult to know with certainty at what point the patient, in this case, became infected with SARSCoV- 2, but taking into account the initial negative test and the positive result (CT=33) of the second test on day 20 of hospital admission, it appears to be a nosocomial infection. Therefore, it is important to review all aspects related to the management of immunosuppressed patients that can be corrected to prevent this type of situation from occurring.
The antibiotic therapy, as well as the dose and frequency in which it should be indicated, is not yet defined in the treatment of Bordetella bronchiseptica infections, so the presence of resistant strains described in the literature should be reviewed. Generally, the isolates reported are sensitive to carbapenemases, fluoroquinolones, and anti-Pseudomonas penicillins; however, due to the aforementioned resistance, the evolution of each case should be monitored individually.10 Taking into account the ability to invade epithelial cells and phagocytes, it is important to include within the antibiotic options to be used, one that achieves sufficient intracellular penetration.16
In this case, the patient was initially treated with cefotaxime; however, after the antibiogram report and little clinical improvement, she was switched to gentamicin, which was effective in controlling the infection.
Finally, it can be concluded that B. bronchiseptica infections should always be evaluated as a marker of immunosuppression, and, in the case that the origin of the immunosuppression is already known, patient management must be done following all the necessary isolation and protection care to avoid other infections. A detailed analysis of the aspects to be improved in patient management is considered of utmost importance to ensure a safer hospital stay for immunosuppressed patients who require it.
Referencias
1. Groner M, Rodriguez A, Doblecki-Lewis S. Bordetella bronchiseptica post-surgical meningitis in an adult. Infect Dis Clin Pract. 2016;24:56-7. [ Links ]
2. Diano D, Allegrini F, Delmonte A, Fausti V, Cravero P, Marcantognini G, Frassineti GL. Bordetella bronchiseptica pneumonia in a patient with lung cancer; a case report of a rare infection. BMC Infect Dis. 2017 Sep 25;17(1):644-9. [ Links ]
3. Dworkin MS, Sullivan PS, Buskin SE, Harrington RD, Olliffe J, MacArthur RD, Lopez CE. Bordetella bronchiseptica infection in human immunodeficiency virus-infected patients. Clin Infect Dis. 1999 May;28(5):1095-9. [ Links ]
4. Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005 Apr;18(2):326-8. [ Links ]
5. Woolfrey BF, Moody JA. Human infections associated with Bordetella bronchiseptica. Clin Microbiol Rev. 1991 Jul;4(3):243-55. [ Links ]
6. Radcliffe C, Lier A, Doilicho N, Parikh S, Kaddouh F. Bordetella bronchiseptica: a rare cause of meningitis. BMC Infect Dis. 2020;20(1):922-6. [ Links ]
7. Shimoni Z, Niven M, Mosenkis M, Greif J. Fatal pneumonia due to Bordetella bronchiseptica. Isr Med Assoc J. 2000 May;2(5):402-3. [ Links ]
8. Echeverri-Toro L, Arango A, Ospina S, Agudelo C. Bacteriemia recurrente por Bordetella bronchiseptica en un paciente con trasplante de medula ósea [Bordetella bronchiseptica recurrent bacteraemia in a patient with bone marrow transplantation]. Biomedica. 2015 Jul- Sep;35(3):302-5. [ Links ]
9. Monti M, Diano D, Allegrini F, et al. Bordetella bronchiseptica pneumonia in a patient with lung cancer; a case report of a rare infection. BMC Infect Dis. 2017;17(1):644-9. [ Links ]
10. Gupta S, Goyal P, Mattana J. Bordetella bronchiseptica pneumonia a thread in the diagnosis of human immunodeficiency virus infection. IDCases. 2019 Feb 19;15:509-16. [ Links ]
11. Clements J, McGrath C, McAllister C. Bordetella bronchiseptica pneumonia: beware of the dog! BMJ. 2018 Apr 27;2018:588-93. [ Links ]
12. Berkowitz DM, Bechara RI, Wolfenden LL An unusual cause of cough and dyspnea in an immunocompromised patient. Chest. 2007;131:1599-602. [ Links ]
13. Yacoub AT, Katayama M, Tran J, Zadikany R, Kandula M, Greene J. Bordetella bronchiseptica in the immunosuppressed population - a case series and review. Mediterr J Hematol Infect Dis. 2014 Apr 7;6:31-6. [ Links ]
14. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28; 395(10229):1054-62. [ Links ]
15. Nagarakanti S, Bishburg E. Coinfection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Bordetella bronchiseptica Pneumonia in a Renal Transplant Patient. Cureus. 2021;13(2):113-7. [ Links ]
16. Papasian CJ, Downs NJ, Talley RL, Romberger DJ, Hodges GR. Bordetella bronchiseptica bronchitis. J Clin Microbiol. 1987 Mar;25 (3):575-7. [ Links ]
Received: May 06, 2021; Accepted: August 19, 2021











 text in
text in 



