Introduction
Endodontic infections are caused by microorganisms that colonized the root canal system (RCS) (1). The root canal treatment (RCT) involves a chemo-mechanical debridement of the RCS (2, 3) by eliminating the biofilm and inflamed pulp tissue (4). Hence, the main aim of the RCT is to optimize the root canal disinfection (2, 5) in order to eliminate bacteria (2) and prevent re-infection (5); In spite of mechanical instrumentation and disinfection, microorganisms remain (6) which proves that the sterilization of the root canal system is impossible to achieve even with current techniques (4).
Due to its antimicrobial and tissue dissolving properties (1, 7) Sodium hypochlorite (NaClO) is the most common disinfection solution used in endodontics (1, 4, 7), it is a proteolytic agent with a wide non-specific bactericidal effect (4). Even though NaClO has many ideal properties, it has some limitations (8); it is ineffective in smear layer removal; is corrosive; it may cause discoloration; has unpleasant odor (8); and when is used as a final rinse, bonding of the sealer to the dentin may be altered (8). Also there is concern about its possible adverse biological effects (7), since it has been shown to have mutagenic activity in both bacterial and mammalian cells in vitro; it shows a potential genotoxic effect in human cells (9); also it has been proven that NaClO may irritate the skin, cause burning, pain, inflammation and blisters (9); there are potential sequelae following ingestion of NaClO, like bleeding, perforation, scarring and stricture formation following corrosive injury to the mouth, throat, esophagus and stomach (9). Considering the limitations and risks related with NaClO, countless compounds in aqueous solution have been suggested as root canal disinfection solution, including inert substances or highly toxic and allergenic biocides (5).
It is necessary to propose and evaluate alternatives to be used as an endodontic disinfection solution, the new proposals should have most of the ideal properties of an endodontic disinfecttion solution (5) and must be tested. Hyperosmotic solutions based on sodium chloride and potassium sorbate has been proven as endodontic disinfection solution, showing an adequate antimicrobial effect against endodontic microorganisms in planktonic (10, 11) and biofilm forms (11), it has been proven that this solutions could be used as potential endodontic disinfection solution or as a complement during the RCT (10, 11). This study evaluated the genotoxicity effect of a hyperosmotic solution that previously showed effectiveness against pathogenic endodontic microorganisms (10, 11).
Methods
This project was approved by the Research Ethics Committee of the Stomatology Faculty, UASLP, code CEI-FE-028-2020.
In a 24-well culture plate and over circular coverslips at the bottom of 24-well culture plate, ATCC primary PCS-201-010 of Dermal fibroblast Normal human neonatal cells were seeded and incubated for 24 h at 37°C in a 5% CO2 atmosphere with Dulbecco's Modified Eagle's Medium (DMEM) enriched with Fetal Bovine Serum (FBS) 10% and antibiotic-antimycotic solution (Sigma- Aldrich) 10.0000 units penicillin, 10 mg and 25 μg Streptomycin B (Str) 1%. After incubation (24 h) the culture medium was replaced with different endodontic disinfection solutions (experimental groups): hyperosmotic solution 30% (450 μL of hyperosmotic solution + 1050 μL of culture medium (DMEM + FBS 10% + Str 1)); hyperosmotic solution 100% (1500 μL of hyperosmotic solution); NaClO 1% (270 μL of NaClO 5.25% + 1230 μL of culture medium (DMEM + FBS 10% + Str 1%)); Cisplatin (1500 μL) (5 mg/mL) (positive control group); culture medium (1500 μL) (DMEM+ FBS 10% + Str 1%) (negative control group). Each solution was placed for 15 seconds, then a PBS wash was performed.
All groups were evaluated by Micronucleus, Tunel and Mitotracker assay (each experiment was performed by triplicated).
Micronucleus assay
After the exposure time with the different irrigating solutions and PBS wash, 1500 μL of culture medium (DMEM + FBS 10% + Str 1%) were placed in each well and cultured for 24 h, at 37°C in a 5% CO2 atmosphere.
The culture medium was removed from the 24-well plates and an PBS wash was carried out; then 5 mg/mL of Cytochalasin B in culture medium (5000 μL) (DMEM + FBS 10% + Str 1%) was incubated for 72 h at 37°C in a 5% CO2 atmosphere; then a wash was performed with 300 μL of sterile PBS to eliminate non-viable cells; 500 μL of KCl (0.075 %) were added for 1 minute; cells were fixed by washing with a solution of methanol + acetic acid (3:1), then the coverslips were stained with 300 μL of acridine orange for 1 minute; the samples were washed with Sörensen's Buffer; sterile PBS was placed and the coverslips were mounted and were observed under a Fluorescence Microscope at 40x. Also, the Proliferation Index (PI) was evaluated.
Tunel assay
After the exposure time with the different irrigating solutions and PBS wash, 1500 μL of culture medium (DMEM + FBS 10% + Str 1%) were placed in each well and cultured for 24 h, at 37°C in a 5% CO2 atmosphere.
The culture medium was removed from 24-well plates and a PBS wash was carried out; then was placed 300 μL of formaldehyde (4%) for 25 minutes at 4°C; then a PBS wash was carried out; after, was added triton X-100 (0.2%) for 5 minutes, then a PBS wash was performed; the cells were covered with 100 μL of equilibration buffer for 10 minutes; 50 μL of rTdT incubation buffer was added to each of the wells without allowing the cells to dry out. Subsequently, the slides were covered with circular plastic coverslips and were incubated for 60 minutes; then 200 μL of reagent solution 20X SSC 1:10 were placed in each well for 15 minutes; a PBS wash was performed, and the cells were stained with 40 μL of Propidium Iodide (1:1000); then the samples were observed by a Confocal Laser Scanning Microscope (CLSM Leica) at 520 nm wavelength.
Mitotracker assay
The mitochondrial status was evaluated though the Mitotracker Deep Red Kit (Thermo Fisher). In dark conditions a preparation of a Working Solution (WS) was carried out at a concentration of 1 μM DMSO, then a second WS (1:3) was carried out; then 200 μL of second WS were placed in each well and incubated for 30 minutes in dark conditions; after, a PBS wash was performed, and the coverslips were mounted and evaluated by CLSM (485 nm wavelength).
Results
Micronucleus assay
To perform the analysis 2000 cells per group were counted (only fibroblasts with intact nuclear and cytoplasmic membranes were considered).
Statistical analysis was performed using Sigma Plot Software. Data according to Micronucleus Percentage (%MNC) and Proliferation Index (%IP) were compared by One-Way Analysis of Variance. The normality of the data was analyzed using the Shapiro-Wilk test. The data were plotted by means (Figure 1).
The Micronucleus Assay demonstrated a significant increase in the frequency of Micro- nucleus with the addition of sodium hypochlorite 1%, corroborating the genotoxic potential of sodium hypochlorite in human fibroblasts; on the other hand, in the current study the hyperosmotic solution (30%/100%) show no genotoxic potential.
A statistical difference (p<0.05) was showed between the negative control and sodium hypochlorite 1%; however, there was no statistically significant difference between hyperosmotic solution (30%/100%) compared to the negative control.
Tunel assay
The results for the TUNEL Assay demonstrated that both, NaClO and hyperosmotic solution produce apoptosis in contact with human fibroblasts, with no significant differences between groups. Based on results, both solutions must be avoided to be in contact with human cells (Figure 2).
Mitotracker assay
The current study showed that the mitochondria function of cells exposed to hyperosmotic solution (30%/100%) remains normal; also, when cells were exposed to NaClO 1% there was a decrease in the number of functional mitochondria, meaning that the metabolic function of cells decreased (Figure 3).
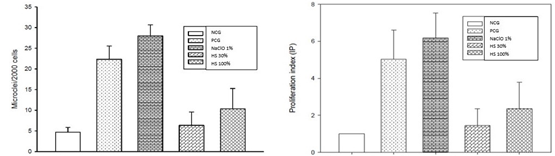
Figure 1 Proliferation Index (IP). NCG=Negative Control Group; PCG=Positive Control Group (CisPlatin); NaOCI=Sodium Hipoclorite 1%; Hyperosmotic Solution 30%; Hyperosmotic Solution 100%. A statistical difference was showed between NCG and NaOCI 1%; there was no statistically significant difference between HS (30%/100%) compard to NCG.
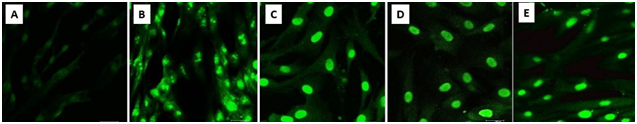
Figure 2 Tunel Assay. CLSM images A) Negative Control Group. 40X. Fibroblasts present nuclear material fragmented, compatible with an apoptotic state. B) Positive Control Group. 40X. Fibroblasts present a normal nuclear structure. C) NaCIO 1% Group. 40X. Fibroblast present nuclear material fragmented, compatible with an apoptotic state. D) Hyperosmotic Solution 100% Group. 40X. E) Hyperosmotic Solution 30% Group. 40X. The fibroblasts present an apoptotic state.
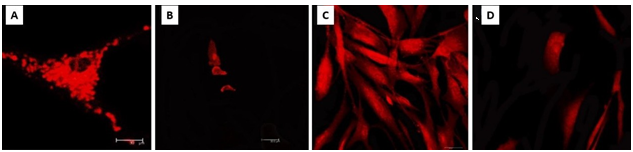
Figure 3 Mitotracker Assay. A) Negative Control Group. 40X. Normal amount of mitocondrial structures and normal mitocondrial morphology, showing an adequate mitochondrial potential. B) NaCIO 1% Group. Amount of mitocondrial structures is reduced in comparation with NCG, low mitocondrial potential and mitocondrial anormal morphology. C) Hyperosmotic Solution 30% Group. Normal amount of mitocondrial structures and normal mitocondrial morphology, showing and adequate mitochondrial potential. D) Hyperosmotic Solution 100% Group. Normal amount of mitocondrial structures and normal mitocondrial morphology, showing and adequate mitochondrial potential.
Discussion
The present study analyzed the genotoxicity of a hyperosmotic solution, which already demonstrated an adequate antibacterial effect against E. faecalis in planktonic (10, 11) and biofilm forms (11).
The long-term success of the endodontic treatment is related with different factors, such as shaping, cleaning and tridimensional sealing of the root canal system (12), also, there are no doubt that microorganisms are the main cause of endodontic failure (5); with such a complex and dynamic microbial environment of the RCS (7), selection of an effective antibacterial (7) and chemical (4) agent to use during treatment is critical (4,7), since the main aim of RCT is to eliminate bacteria (2) and achieve an adequate root canal disinfection (5).
NaClO is recommended as the main endodontic disinfection solution due to of its ability to dissolve organic matter together with its antimicrobial action (13), it has a wide activity against both gram negative and positive bacteria and has the strongest antibacterial and antifungal activity among canal endodontic disinfection solutions. Furthermore, it is the only endodontic disinfection solution with the ability of destroying the microbial biofilm (13). Nevertheless, it has been shown that use of NaClO, may result in the disintegration of the organic dentine matrix (1), and affect mechanical dentin properties (8); it produce some degree of postoperative pain or discomfort following traditional endodontic irrigation (8), particularly if extruded beyond the apical foramen (1); also, ingestion of small volumes of sodium hypochlorite is associated with burns on mouth and throat, gastrointestinal irritation, nausea/vomiting, abdominal and retrosternal pain, diarrhea, dysphagia, stridor, drooling, abdominal pain and dyspnea (9); the aspiration of it may lead to pulmonary complications such as acute respiratory distress syndrome (ARDS) (9). Other complications include maxillary sinus incidents, severe pain, cellulitis, life-threatening events, permanent facial disfigurement, permanent nerve damage, and secondary infection (8). Also, in vitro genotoxicity and cytotoxicity of NaClO has been proved in previous reported literature, Ugur et al. evaluate the toxicity and oxidative DNA damage of sodium hypochlorite and showed that there was a statistically significant decrease in cell viability in the NaClO (14).
Marins et al. tested the effect of NaClO at different concentrations over murine fibroblasts, and the results showed that exposure to 2.5% and 5% NaClO resulted in a significant cytotoxic effect in a dose-dependent manner over cells (15). In the study of Gül et al. were evaluated the effect of NaClO over human lymphocytes, the results indicate that NaClO significantly increased the frequency of micronucleus in a dose dependent manner, also there was a significant correlation between NaClO concentration and chromosomal aberration, micronucleus frequency, necrotic cells, apoptotic cells and binucleated cells (16).
Due to limitations and negative effects related with NaClO is necessary to propose and evaluate new endodontic disinfection solution as an alternative to current solutions. Hyperosmotic solutions have an adequate antibacterial effect (10, 11), the antibacterial effect of hyperosmotic solutions is based on the fact that hyperosmotic stress causes loss of cellular water and cellular contraction, elevates the intracellular ionic force and generates macromolecular overcrowding and protein denaturation, thus inducing cellular plasmolysis (11). Persistent infection of the root canal due to the presence of resistance bacterial species (2), such as E. faecalis (2, 4) has always been one of the most important reasons for endodontic treatment failure (2). This microorganism is a gram-positive facultative anaerobic bacterial species, which is the most common species in resistant root canal infections (2, 4), so new endodontic disinfection solutions must be effective against E. faecalis. The current hyperosmotic solution already has been proved to have an adequate antibacterial effect against E. faecalis in planktonic (10, 11) and biofilm forms (11); another potassium sorbate solutions has been tested and evaluated in combination with sodium chloride and was reported a synergistic combination against E. faecalis biofilm (17).
Conclusions
According to the results of this study the hyperosmotic solution did not produce alterations in the function of mitochondria, also, based on Micronucleus Assay it has not shown genotoxic effects over human fibroblasts; nevertheless the hyperosmotic solution could produce apoptosis on human fibroblast based on Tunel Assay, based on this results the hyperosmotic solution is a safe potential endodontic disinfection solution or complement for root canal treatment disinfection, but still must be avoid to extrude the solution to periapical tissues due to potential apoptosis. Further evaluations should be performed to corroborate all data.
Author contribution statement
Conceptualization and design: C.M.M.F., R.O.R.
Literature review: C.M.M.F., R.O.R.
Methodology and validation: A.J.P.G., D.M.E.G.
Formal analysis: A.I.M.R.,
Investigation and data collection: J.M.C., R.O.R.
Resources: A.J.P.G., D.M.E.G.
Data analysis and interpretation: A.I.M.R., J.M.C.
Writing-original draft preparation: R.O.R. F.J.G.C.
Writing-review & editing: F.J.G.C., J.M.C.
Supervision: R.O.R.
Project administration: A.I.M.R., A.J.P.G.
Funding acquisition: A.J.P.G.















